There is evidence that an emerging type of immunotherapy called TIL therapy may be successful in treating more solid tumors.
Research published in Cancer Discovery shows this treatment works in some patients with non-small cell lung cancer. The lead researcher of the multicenter clinical trial was Memorial Sloan Kettering Cancer Center (MSK) lung cancer specialist Adam Schoenfeld, MD.
“The patients who came to us in this trial were very sick and had often exhausted all other options,” says Dr. Schoenfeld, a thoracic oncologist, cellular therapist, and early drug development specialist. “The fact that 21% of the non-small cell lung cancer patients responded to TIL therapy is significant. Some patients remained free of cancer relapse for more than two years after the treatment.”
TIL Therapy Clinical Trial Results for Non-Small Cell Lung Cancer Patients
TIL (pronounced “till”) stands for tumor-infiltrating lymphocytes, a specialized type of white blood cell. The approach involves removing TIL cells from the tumor, growing them into large numbers, and then putting them back into the patient. There, the TIL cells can seek out and destroy cancer cells anywhere in the body.
The phase 2 clinical trial published in Cancer Discovery involved 28 patients treated at several hospitals in the United States and Europe.
- All the patients had late-stage non-small cell lung cancer that had metastasized (or spread) and had become resistant to multiple other therapies, including checkpoint inhibitors, another type of immunotherapy.
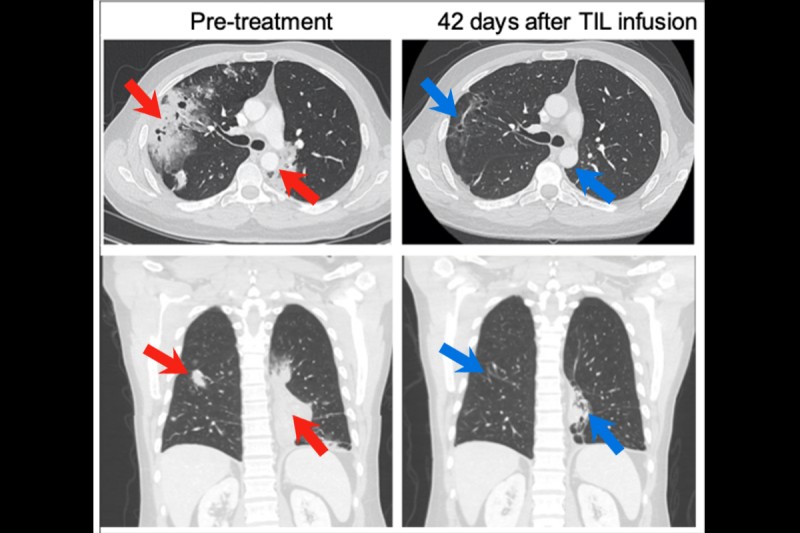
- Overall, 6 of 24 patients whose outcomes could be evaluated responded to the TIL treatment, meaning that the TIL cells were active at destroying their cancer.
The scans of patients before and after the treatment are striking.

TIL therapy was recently approved to treat metastatic melanoma that has not responded to other treatments. That treatment, called lifileucel (Amtagvi™), became the first cell therapy approved by the Food and Drug Administration to treat a solid tumor.
“TIL therapy has been studied in melanoma for many years, but until very recently no one had ever shown the treatment could be effective for lung cancer,” Dr. Schoenfeld says. “This study is significant because it was one of the first times anyone demonstrated that generating TIL cells from lung tumors was feasible and that these cells could generate anti-tumor activity.”
He explains that since this trial was launched at MSK four years ago, other trials have shown that for patients earlier in the course of disease — those who have not developed resistance to checkpoint inhibitors — the response rates are about twice as high. Those findings were presented at the World Conference on Lung Cancer in September 2023.
How TIL Therapies Work
TIL therapies take advantage of the immune system’s natural ability to recognize and attack cancer. They are in a class of treatments known as cell therapies — the patient’s own cells fighting cancer. Cell therapies are often called “living drugs” because once in the body, they continue to circulate through the bloodstream and fight cancer.
For many people with blood cancer, other cell therapies called CAR T have successfully controlled the cancer. TIL is another form of cell therapies for treating solid tumors, which make up the majority of cancers. This is why oncologists are excited about the prospects of TIL therapy.
How TIL Therapy Is Given to Lung Cancer Patients
TIL therapy involves several steps:
- First, patients must have surgery to remove one of their tumors to extract the TIL cells. The tumor does not need to be in the original location where the cancer developed; the TIL cells can be collected from a lymph node or another organ where the cancer has spread.
- While patients are recovering from surgery, the TILs are grown in a lab.
- About a month after the surgery, patients go back to the hospital to receive chemotherapy to tamp down their immune system and prepare it to receive the TILs.
- Then the TIL cells are infused into the bloodstream to circulate and track down cancer cells.
- Patients also receive an immune-boosting drug called interleukin-2 to help the TIL cells establish themselves and grow.
Making TIL Therapy Safer and Even More Effective
TIL therapy has serious side effects, including low blood counts that make patients weak and susceptible to infections. However, the majority of side effects are caused by the chemotherapy and the interleukin-2, not by the TIL cells. Interleukin-2 can also cause severe side effects, such as shortness of breath, heart problems, and kidney injury, if not monitored closely in the hospital.
To make TIL treatment safer, next-generation therapies are being developed that allow patients to avoid treatment with interleukin-2. For example, other potentially safer and more effective interleukins such as interleukin-15 may be engineered into the patients’ TIL cells while they are in the lab, limiting their effects only to the cancer and not the rest of the body.
TIL Therapy for Lung Cancer Clinical Trials at MSK
Dr. Schoenfeld is leading a phase 2 trial for one of these next-generation TIL therapies, called OBX-115, in lung cancer. OBX-115 is also being studied for melanoma at several cancer centers, including at MSK, led by melanoma oncologist and cellular therapist Alexander Shoushtari, MD. Notably, MSK is currently the only hospital offering it for lung cancer.
Dr. Schoenfeld is also looking for other ways to make the therapy less difficult for patients. Some research has suggested that it may be possible to lower the doses of chemotherapy given before the infusion of the TIL cells, further reducing side effects of the treatment.
“TIL therapy has the potential to benefit so many people with lung cancer,” Dr. Schoenfeld says. “This is only the beginning. As we can find ways to make it safer, TIL and other cell therapies will offer an important new therapy for a group of patients who have had little success with other treatments.”

