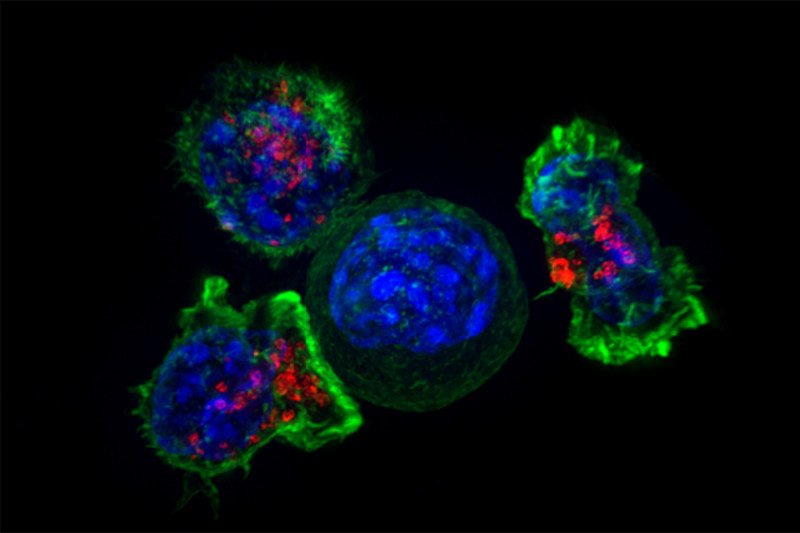
Immune T cells surround a cancer cell. Researchers are investigating ways to stimulate these immune cells to fight sarcoma. (Source: Alex Ritter, Jennifer Lippincoll Schwartz and Gillian Griffiths, National Institutes of Health)
Update: This story was originally published on July 28, 2016, and has been updated with new information.
Immunotherapy, which harnesses the power of the immune system to fight disease, has recently shown impressive results in the treatment of multiple cancers. The Food and Drug Administration’s approval of a class of drugs called checkpoint inhibitors has dramatically improved therapeutic options. People with melanoma, lung cancer, kidney cancer, bladder cancer, leukemia, and other cancers may benefit from these drugs.
Researchers at Memorial Sloan Kettering are hoping that this immunotherapy success can be applied to the treatment of sarcomas as well. These rare cancers grow in the body’s connective tissues, including fat, blood vessels, nerves, bones, muscles, and cartilage.
We spoke with MSK medical oncologist Sandra P. D’Angelo about the current state of research, including which clinical trials at MSK are under way or planned to open soon.
Why use immunotherapy as a treatment for sarcoma?
Sarcoma is a devastating disease for which we need more-effective therapies. Sarcoma has more than 50 distinct subtypes, and the threat of metastasis can be high. Depending on the type of sarcoma and its initial size, the disease can spread in some patients who are diagnosed. Men and women whose cancer has spread to other parts of the body often respond to treatment but the duration of benefit can sometimes be short and the side effects can sometimes be tough.
With conventional treatments such as chemotherapy, or even newer targeted therapies, we try to treat the tumor. But it will likely prove difficult to develop a single therapy that would work across all of the sarcoma subtypes. Each of these diseases may behave differently and require distinct treatment approaches.
Immunotherapy is an appealing option because it’s designed to empower the immune system to fight many different types of cancer, not just one. My colleague Jedd Wolchok and I worked on immunotherapy treatment for melanoma. He takes the view that it’s often better to treat the person and let their own body treat the tumor.
What types of immunotherapy are now being investigated for sarcoma?
The main approaches involve checkpoint inhibitors and adoptive T cell therapy. Checkpoint inhibitors are drugs that block specific proteins on the surface of immune T cells. This releases a natural brake on the immune system, allowing it to attack the cancer. Adoptive T cell therapy involves removing T cells from patients and modifying the cells in a way that enables them to recognize and attack specific molecules on the surface of cancer cells. MSK has led the way in using both of these approaches to treat cancer.
How is checkpoint inhibitor–based immunotherapy being tested against sarcoma at MSK?
The checkpoint inhibitors we are investigating include the drugs ipilimumab (Yervoy®), nivolumab (Opdivo®), and pembrolizumab (Keytruda®), which we’ve already seen be effective in multiple cancers.
Ipilimumab targets a protein called CTLA-4. In earlier clinical studies, it seemed to have a very minimal effect when used alone against selected sarcomas. The latter drug, nivolumab, targets a different protein, called PD-1. We were struck by the deep and rapid responses in people with melanoma when ipilimumab was combined with nivolumab. We think combination immunotherapy will benefit more people with sarcoma. Much of our efforts are focused on identifying the best drugs to combine for patients.
I led a national phase II clinical trial testing the combination of these drugs in people with metastatic sarcoma. We found that nivolumab and ipilimumab are effective in certain sarcoma subtypes. These include undifferentiated pleomorphic sarcoma and myxofibrosarcoma (both types of liposarcoma) as well as leiomyosarcoma, and angiosarcoma. The tumor responses appeared to be similar to those seen with standard chemotherapy. In addition, the combination of these two drugs was found to be very safe and tolerable. The findings were published in January 2018 in the journal Lancet Oncology. They support future studies of this drug combination for people with specific metastatic subtypes. We are continuing to focus on identifying sarcoma biomarkers that allow us to predict who will respond well to this type of immunotherapy.

We’re also exploring therapies that combine checkpoint inhibitors with new drugs that boost the immune response in other ways. For example, there is an ongoing effort combining nivolumab with NKTR-214. This drug is a modified form of a protein called interleukin-2, which is made by the immune system. NKTR-214 is designed to trigger other cells in the immune system to attack cancer cells. The goal is to see if this combination will increase the likelihood of an immune response for metastatic or locally advanced sarcoma.
In addition, there’s an upcoming study combining pembrolizumab with a drug called epacadostat that will be conducted in collaboration with medical oncologist Ciara Kelly. Tumors often can produce an enzyme called IDO1 to avoid the immune system. Epacadostat blocks this enzyme to help the immune system hit the tumor with its full force.
Most recently, in collaboration with Dr. Kelly, we conducted a clinical trial utilizing TVEC, an attenuated herpes virus injected directly into the sarcoma tumor, in combination with pembrolizumab. This trial just completed patient accrual, and we are currently analyzing the results.
What about the use of adoptive T cell therapy for sarcoma?
We have been collaborating with a company to engineer T cells to fight synovial sarcoma. This type of sarcoma has a specific protein called NY-ESO-1 that is not on other cells. Because of that we are able to target the cancer cells selectively. The treatment is being tested in a pilot study at MSK. The trial involves removing T cells from a person with synovial sarcoma, engineering the cells to recognize the NY-ESO-1 protein, and then giving them back to the person in large numbers.
How Clinical Trials Can Benefit You
As of November 2017, 37 patients have received these modified T cells in multiple groups with variations to the treatment approach. Overall, the response rate has been promising. There are ongoing efforts to optimize this treatment approach and determine the best strategy moving forward. We are now in the process of leading a similar effort for people with myxoid liposarcoma. Their tumors also express NY-ESO-1. This trial is actively enrolling patients.
Beyond this specific approach, we are working with MSK investigator Michel Sadelain on a type of adoptive T cell therapy called chimeric antigen receptor (CAR) T cell therapy. CAR T has demonstrated remarkable results in people with chemotherapy-resistant leukemia. We are trying to develop CAR T cells that will target a protein that’s on the surface of many sarcomas. That’s a big project that we’ve been working on for several years. We hope to launch a clinical trial testing this approach in the near future.
What is the biggest challenge for using immunotherapy against sarcoma?
The major hurdle is identifying the right strategies for specific subtypes. It’s difficult to know what will work in a particular sarcoma. The cells all look different under a microscope and have different mutations. There are ongoing efforts to identify sarcoma biomarkers that can help us predict whether a therapy will be effective.
The important point is that there is great potential and hope for immunotherapy to have some effectiveness against sarcoma. Both checkpoint inhibitors and CAR T cell therapy have demonstrated success in other cancer types. We hope to continue to figure out ways to extend this benefit to people with sarcoma.