For the first time, a team of Memorial Sloan Kettering investigators has synthesized erythropoietin (also known as EPO), the hormone that controls the production of red blood cells. The researchers, led by Samuel J. Danishefsky, a member of the Molecular Pharmacology and Chemistry Program in the Sloan Kettering Institute, have also shown that the molecule they produced in the laboratory functions in the manner of natural erythropoietin.
A culmination of ten years of work, the accomplishment is a significant advance in the chemical synthesis of complex biological molecules from basic building blocks: simple amino acids and carbohydrates. Furthermore, the researchers believe that being able to synthesize this vital molecule will enable them to learn more about how EPO functions as well as how the body makes proteins with complicated structures.
“This work opens up a new chapter in protein chemistry because we can now use chemical synthesis to make very complex molecules that have previously been thought to be producible only by nature,” says Dr. Danishefsky, senior author of the study published online September 25 in the chemistry journal Angewandte Chemie. “Being able to make large biological molecules such as hormones in the laboratory can lead to many new avenues of research.”
A Hormone Essential to Life
Erythropoietin, which is produced in the kidneys, is critical to life because it controls the production and performance of red blood cells. As a commercial drug, EPO — which is currently manufactured in cell cultures — is used primarily for the treatment of anemia in people with chronic kidney disease and in patients who have undergone chemotherapy or radiation therapy for cancer.
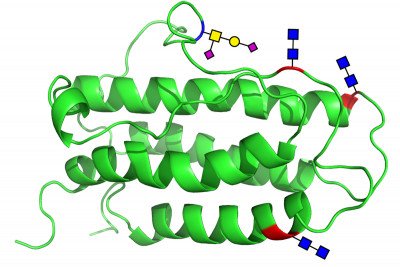
Erythropoietin as it is now produced is not actually one compound but a mix of a large family of molecules. Known as glycoproteins, the structures are composed of a protein with four sectors of carbohydrates attached, known as carbohydrate domains. The protein portion is always the same, as are the locations where the carbohydrate domains attach.
The variation in these structures is also seen in the vast range of different carbohydrates found in natural EPO. In this new study, however, the investigators were able to make for the first time a pure EPO, in which all the carbohydrates were specifically selected.
Confirmation That the Molecule Functions
After the molecule was synthesized, the investigators had to determine whether it would induce the formation of red blood cells. Study co-author and cell biologist Malcolm A. S. Moore and his laboratory at Memorial Sloan Kettering combined stem cells from umbilical cord blood with the pure hormone synthesized in the Memorial Sloan Kettering laboratory and confirmed that the molecule functioned as expected. “We showed that the synthesized erythropoietin was causing the stem cells to differentiate into red blood cells and proliferate,” Dr. Moore explains.
In addition, Ronald Hendrickson, Head of Memorial Sloan Kettering’s Proteomics and Microchemistry Core Facility, used a highly sophisticated version of a technique called mass spectrometry, which separates the parts of a molecule based on their masses, to verify that the structure created by the Memorial Sloan Kettering team was the same as the natural glycoprotein.
Stepping Stone to Additional Studies
Part of the team’s motivation for generating fully synthetic erythropoietin came from the desire to gain access, for the first time, to a pure form of the natural molecule. The ability to generate pure versions of EPO will enable scientists to make numerous versions of the molecule and study whether differences in the sizes of the carbohydrate domains attached affect how the glycoprotein induces the production of red blood cells.
“This is a major development in the field of chemistry,” Dr. Moore says. “It will help us immensely in understanding the complexity of this molecule.”

