When Karuna Ganesh came to Memorial Sloan Kettering as an oncology fellow in 2013, she already knew she wanted to do research. A physician-scientist by training, she has a passion for seeking answers to fundamental scientific questions. But it was spending time with people with cancer during her first-year rotations that convinced her what her research focus would be.
“It was obvious that metastasis is the number-one most pressing clinical problem in oncology,” she says. “I saw many patients whom we could no longer hope to cure because their cancer had spread.” Ultimately, metastasis is responsible for about 90 percent of cancer deaths.
The question that she and others want to answer is why. Why is it so hard to cure people with metastatic cancer? What is it about metastatic cells that allows them to thwart doctors’ best therapies?
What is cancer metastasis?
Cancer metastasis occurs when cells break off from a tumor, spread through the bloodstream or lymph vessels, and establish themselves in another part of the body. Learn more about how cancer metastasis works.Dr. Ganesh is seeking answers to these questions in the lab of Joan Massagué, Director of the Sloan Kettering Institute. Dr. Massagué has spent the better part of two decades asking what makes metastatic cancer cells tick. He thinks the science is now at an in inflection point.
“We’re at a moment when insights from stem cell biology, tissue regeneration, and immunology are beginning to lift the veil on metastasis,” Dr. Massagué says. “This convergence of knowledge has given us a new way of looking at an old problem.”
From this fresh perspective, metastasis looks a lot like normal growth and repair gone awry. Cancer cells hijack these processes to serve their own ends. While certainly devious, this co-option also means that scientists can use what they’ve learned about normal development and regeneration to better combat cancer.
Borrowing from Regeneration
For all its lethal power, metastasis is actually a very rare event. Although growing tumors shed cancer cells into the circulation continuously, less than 1% of those cells will ever form measurable metastases. In part, that’s because there are so many barriers they must overcome.
“Metastatic cancer cells face major hurdles that normal cells rarely have to face,” Dr. Ganesh says. “They must let go of their friendly tumor environment, travel through the bloodstream or lymphatics, and then take up residence in a completely different organ. That’s an incredibly difficult journey, one that most cancer cells do not survive.”
She and her colleagues are studying each one of these hurdles to find out how a few cancer cells ultimately manage to surmount them all. They’re finding clues by looking at the molecules that metastatic cancer cells produce at different stages of their journey.
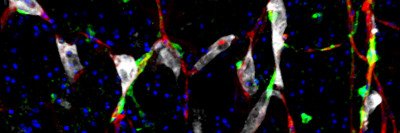
One molecule that stands out is a protein called L1CAM. This sticky molecule is typically found on the surface of neurons during brain development. The cells use it to crawl to the proper location in the brain and sprout branches. But some cancers also produce L1CAM, and when they do, patients have a worse prognosis.
Previous studies from the Massagué lab have shown that L1CAM helps metastatic cancer cells hold on to blood vessels in the brain, where they can hide out and remain undetected for months or years. But newer studies from the lab suggest L1CAM may play a more widespread role in metastasis.
In a recent paper published in Nature Cell Biology, SKI postdoctoral fellow Emrah Er, Dr. Ganesh, and colleagues showed that L1CAM helps cancer cells colonize a new area. Without L1CAM, cancer cells can infiltrate new tissue, but they cannot grow. With L1CAM, they are able to start dividing and produce a full-blown tumor.
Dr. Ganesh has also shown that L1CAM is found on both metastatic colon cancer cells and normal gut cells that are in the process of repairing tissue damage, suggesting a link between regeneration and metastasis.
Efforts are under way in the lab to target L1CAM with drugs, to prevent cancer cells from overcoming what amounts to the last hurdle on the path to creating a new tumor.
Not a Question of Mutation
Scientists used to think that what distinguished a metastatic cancer cell from a nonmetastatic cancer cell was the number and type of genetic mutations it had. The idea was that metastatic potential was something that evolved late, as more and more mutations built up in tumor cells.
But scientists now know this isn’t generally the case. Metastases often share the same set of driver mutations that are present in the primary tumor. This consistent overlap implies that it’s not unique “metastasis mutations” that lead to cancer spread.
“I think that’s the biggest transformation in our thinking about metastasis in recent years,” says Kristian Helin, the new Chair of the Cell Biology Program in SKI. “It means that the potential for metastasis can be present very early on in the development of a tumor.”
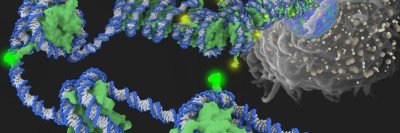
Dr. Helin joined MSK in September. In addition to chairing the Cell Biology Program, he is also directing MSK’s Center for Epigenetics Research. Epigenetics is concerned with how changes in the way that DNA is packaged in a cell influence what genes are turned on or off. Genes that are turned on are said to be “expressed.”
He says you can sometimes predict how likely a cancer is to metastasize by looking at the patterns of gene expression in the primary tumor. This fact provides the basis for genomic tests, such as Oncotype and MammaPrint, that use gene expression profiles to score a tumor’s aggressiveness and likelihood of coming back after surgery.
Analyzing the gene expression profiles of cancer cells has yielded other interesting findings that shed light on cancer’s wiliness. For example, in their gene expression patterns, cancer cells more closely resemble stem cells than fully differentiated cells — say a muscle or immune cell. “That’s been found again and again,” Dr. Helin says, and helps to explain how cancer cells can divide indefinitely, as stem cells do.
The View from a Single Cell
But not every cancer cell in a tumor is capable of metastasis. And that leaves unsolved the nagging question of what makes this subset of cells different.
To analyze gene expression in metastatic cells, SKI researchers are taking advantage of a powerful technique called single-cell ribonucleic acid sequencing (RNA-seq). This approach analyzes the messenger RNA inside of cells to determine which genes are expressed. And it can be done with single-cell precision.
“Without going down to the level of single cells, we aren’t fully able to understand what’s going on and what is driving a particular cancer,” says computational biologist Dana Pe’er, who, in collaboration with the Massagué lab, is using this approach to analyze what is different about metastasis-initiating cells compared to other cells in a tumor.
Dr. Pe’er joined SKI in 2016 as Chair of the Computational and Systems Biology Program as well as Scientific Director of the Alan and Sandra Gerry Metastasis and Tumor Ecosystems Center. She came to MSK in part to apply single-cell sequencing to the toughest problems in cancer research.
In a recent collaboration with SKI Immunology Program Chair Alexander Rudensky, Dr. Pe’er used this technique to study cells taken from human breast tumors, as well as normal breast tissue, blood, and lymph node tissue. They analyzed 45,000 immune cells taken from eight tumors and 27,000 additional immune cells. Their goal was to compare the features of immune cells in tumors to those in normal tissues, to identify which features correlate with disease progression.
“Until very recently, analyzing the data from thousands of cells at the same time would have been a major undertaking,” Dr. Rudensky says. “But thanks to the transformative work that’s been done by Dr. Pe’er’s team, we can start to grow this area of immunology research.”
Identifying differences among immune cells in the tumor area could explain why cancer treatments that harness the immune system, called immunotherapies, don’t always work the same way in each person. Some types of immune cells attack cancer, while others protect tumor cells from harm.
“From this work, we can start to think about how to develop immunotherapy that’s custom-built for people based on their individual tumor microenvironment,” Dr. Rudensky says.
Stealth Mode
Perhaps the most confounding aspect of metastasis is the fact that it can happen years or even decades after a primary tumor is removed. This implies that metastatic cells exist throughout the body but do not always grow right away. Like seeds falling on frozen ground, they may need to find suitable soil before they can germinate.
To persist this long undetected, these cells must be able to avoid being killed by standard cancer treatments like chemotherapy, and also by the immune system.
The main way they do that, SKI researchers have found, is by going into states of periodic dormancy, when they aren’t dividing. Nondividing cells are less likely to be killed by chemotherapy, and are also less likely to be flagged by the immune system as dangerous.
When these cells do wake up and divide, cells of the innate immune system, called natural killer cells, are especially important at keeping them in check.
These discoveries suggest ways to make latent metastatic cells more visible to the innate immune system, so they can’t hide out in the body for so long. That could mean improved immunotherapies for people with cancer.
“I think we’re at the cusp of a new era in understanding more about the cells that cause metastasis,” Dr. Massagué says. “With this convergence of disciplines and technologies like single-cell sequencing, we are finally beginning to understand how these cells differ at the molecular level. Hopefully that will lead to better drugs.”