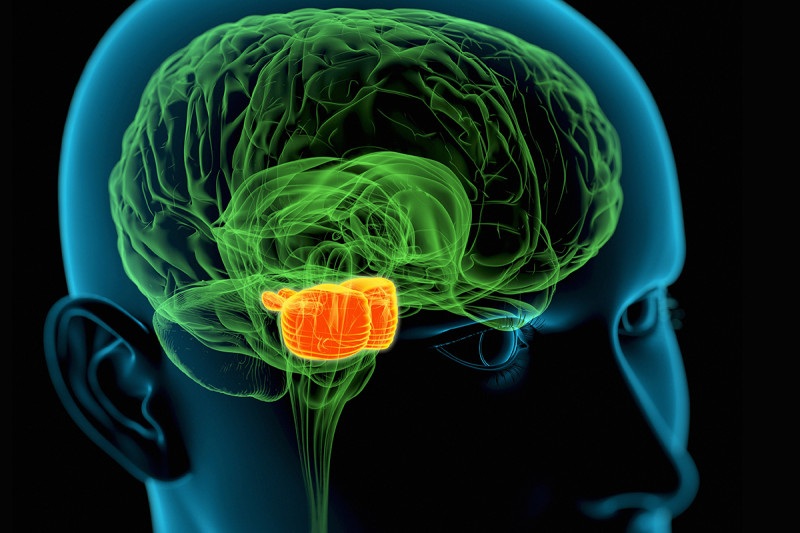
DIPG tumors begin in the middle region (orange) of the brain stem, which regulates many critical body functions. A new study suggests that a method for delivering drugs to these pediatric tumors could be safe and effective. (Credit: Roger Harris/Science Source)
Brain tumors are one of the most difficult cancers to treat because of the blood-brain barrier, which protects the brain against toxins. That barrier also means treatments such as chemotherapy can’t be delivered to tumors in high enough concentrations to be effective. As a result, there has been little to no progress treating tumors deeply embedded in the brain.
Now researchers at Memorial Sloan Kettering Cancer Center (MSK) and Weill Cornell Medicine report hopeful results from a phase 1 study that tested a novel drug delivery technique called convection-enhanced delivery (CED). It slowly infuses drugs directly into the brain, bypassing the blood-brain barrier and targeting specific regions.

Dr. Mark Souweidane
The findings indicate that CED appears safe and effective at distributing a drug throughout a rare and particularly deadly pediatric brain tumor called diffuse intrinsic pontine glioma (DIPG). The median survival for DIPG is typically 8-12 months. Several children in the trial have lived for more than three years after treatment.
The results are published in the journal Neuro-Oncology.
“This is the most exciting thing I’ve done in my career by far,” says Mark Souweidane, MD, a pediatric neurosurgeon at MSK and Weill Cornell who led the study. “I’ve been in this for 30 years, and you just watch these kids die with no alternative. It’s constant, constant turmoil and tragedy. It’s amazing to think you’re on the verge of something big.”
What Is DIPG?
DIPG tumors typically occur in children and begin in the brain stem. This area at the base of the brain regulates many critical body functions, such as breathing, heart rate, and swallowing. DIPG is very difficult to treat because of its location and because the tumor cells can infiltrate normal brain tissue. Surgery is not possible. The only standard option has been radiation treatment, which has a minimal effect.
The new study opens the potential to deliver drugs more efficiently to DIPG tumors — and possibly other tumors located deep in the brain.
CED Slowly Pushes Drugs From Cell to Cell
The convection-enhanced delivery system for DIPG works by slowly infusing the drug through catheters inserted deep into the brain stem. The process lasts up to 12 hours. This extended flow allows the drug to gently push through the fluid compartment between cells in the tumor, as pressure between the cells fluctuates and allows the drug to keep moving through the tissues. As a result, the drug saturates more of the tumor than do conventional techniques.
“With this trial, we have shown that we can use this very powerful drug-delivery platform repeatedly and safely,” Dr. Souweidane says. “The significantly longer survival of several patients is impressive, and we will try to determine why that happened to see if we can make the treatment more effective.”
Previously, CED had been tested primarily on adult brain tumors, such as glioblastoma, another aggressive cancer. Dr. Souweidane says the drug delivery technique is better suited for DIPG because these tumors are smaller and restricted to a tighter area.
Clinical Trial Results Using CED for DIPG in Children
The research underpinning the use of CED for DIPG was an exhaustive effort conducted at Weill Cornell, where Dr. Souweidane is Director of Pediatric Neurological Surgery and Co-Director of the Children’s Brain Tumor Project. The clinical trial, which began in May 2012, took place at MSK.
Earlier results from the trial, published in the journal The Lancet Oncology in 2018, suggested the CED approach was safe. At that time, only 28 children with DIPG had received the treatment. The new results, from 50 patients, provide further proof that the treatment is safe. It also helped researchers determine the optimal dose level.
In the trial, children with DIPG who had already received radiation therapy to the tumor were given a drug called 124I-Omburtamab using CED. This drug consists of an antibody linked to a radioactive substance. The antibody binds to a protein on the surface of brain tumor cells, and the radiation emitted kills the cancerous cells. MSK physician-scientist Nai-Kong Cheung, MD, PhD, a study co-author, created 124I-Omburtamab. The drug has already proven effective in treating metastatic neuroblastoma to the brain.
At seven different dose levels, the delivery method appeared safe in children with DIPG. Researchers determined that 124I-Omburtamab was well distributed through the tumors by tracking the radioactive substance using PET/CT scans and MRI. Most impressively, the investigators were able to prove that drug concentrations in the tumor were up to a thousandfold higher than anywhere else in the body — a remarkable improvement over what is typical with conventional forms of administration.
Impressively, survival data from this limited phase 1 study has resulted in three long-term survivors, the longest exceeding 10 years. These results validated using CED for children with DIPG.
Remaining Challenges
Dr. Souweidane says that data gathered from this trial will guide the next steps in the design of the ongoing and follow-up trial at MSK. How much of the drug made it into the tumor, how long it stayed there, the best ways to image and measure results, and other vital information will be assessed to develop the best therapeutic strategy.
Additional Authors, Funding, and Disclosures
Additional authors on the paper include Evan D. Bander of MSK and Weill Cornell, and Pat Zanzonico, Anne S. Reiner, Nicole Manino, Sofia Haque, Jorge A. Carrasquillo, Serge K. Lyashchenko, Sunitha B. Thakur, Jason S. Lewis, Maria Donzelli, Steven M. Larson, Kim Kramer, Neeta Pandit-Taskar, and Ira J. Dunkel — all of MSK.
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Y-mAbs provided funding and support for the clinical trial.
Dr. Nai-Kong Cheung has intellectual property rights and interests related to 124I-Omburtamab, and also has equity interests in Y-mAbs Therapeutics. Researchers at MSK, including Dr. Cheung, developed 124I-Omburtamab, and MSK licensed this intellectual property to Y-mAbs Therapeutics. MSK has institutional financial interests related to 124I-Omburtamab and Y-mAbs Therapeutics.
Additional research support was provided by:
Cristian Rivera Foundation, McKenna Claire Foundation, The Lyonhearted Foundation, Christian Koehler Foundation, Brooke Healey Foundation, Fly a Kite Foundation, Children’s Brain Tumor Family Foundation, Joshua’s Wish, Lily LaRue Foundation, and Alex Lemonade Stand Foundation for Childhood Cancer.
Read the study: “Phase 1 dose-escalation trial using convection-enhanced delivery (CED) of radioimmunotheranostic 124I-Omburtamab for diffuse intrinsic pontine glioma (DIPG),” Neuro-Oncology, DOI: 10.1093/neuonc/noaf039