Robin Bankins says the first time she spoke with MSK’s Dr. Eli Diamond about treating her histiocytosis, she trusted him right away.
- Based a clinical trial conducted at MSK, the FDA has approved the targeted drug cobimetinib (Cotellic®) for treating a rare group of diseases known as histiocytoses, or histiocytic neoplasms in adults.
For years, Robin Bankins had chronic sinus pain — one of the effects of Rosai-Dorfman disease, a rare disorder that caused a type of white blood cells called histiocytes to build up in her sinuses and throat. Doctors near her home in Baltimore tried to relieve her symptoms with steroids, but their side effects made her feel awful.
When Robin’s airway accumulated so many histiocytes that she had trouble breathing, she was told she needed an operation that would require a tracheotomy tube for at least a month afterward. The 61-year-old grandmother from Baltimore was scared, and she decided to consult Memorial Sloan Kettering Cancer Center (MSK) for a second opinion.
During Robin’s first meeting with MSK neuro-oncologist Eli Diamond, MD, which was a telemedicine visit, she says he told her: “’You’re not having that surgery. We’re not going to treat just one part of your disease. We’re going to treat all of it.’ ”
“I felt so encouraged, and I trusted him right away,” she adds.
On October 28, 2022, the U.S. Food and Drug Administration (FDA) approved the drug that Robin has been receiving for nearly two years, cobimetinib (Cotellic®), for treating Rosai-Dorfman and other forms of histiocytosis.
Cobimetinib is an important example of how a drug that successfully treats one kind of cancer can be used to treat another, because a single mutation can drive different diseases. Cobimetinib was previously approved for people with melanoma. The drug, taken daily as a pill, targets the mutated MEK protein, which causes the body to produce too many histiocytes.
Call 800-525-2225
Available 24 hours a day
What Is Histiocytosis?
Sometimes called histiocytic neoplasms, histiocytoses are a diverse group of diseases caused by an overproduction of histiocytes. They include:
- Erdheim-Chester disease, which is more common in adults and can affect any part of the body, including the brain.
- Langerhans cell histiocytosis, which is more common in children and is most frequently a mild illness affecting the skin or only a few bones.
- Rosai-Dorfman disease, which is usually found in children and typically causes swollen lymph nodes. It can happen in people with autoimmune diseases or blood cancers, but it is not considered to be cancer.
“There has always been an unmet need for treating people with histiocytosis,” Dr. Diamond says. “We are thrilled that with this approval patients will now have access to a viable treatment option.”
MSK Trial Leads to Drug Approval for Histiocytosis
The FDA’s approval of cobimetinib for histiocytosis was based on a phase 2 clinical trial done solely at MSK. This is rare in the field of cancer research, because clinical trials that lead to drug approvals are usually conducted at multiple hospitals. People with histiocytosis travel from all over to be cared for by Dr. Diamond and his team.
The original trial enrolled 26 patients; 10 more were later added.
- More than three-quarters of the patients saw marked improvements in their condition.
- More than half had a complete response, which means that their disease was no longer detectable on scans.
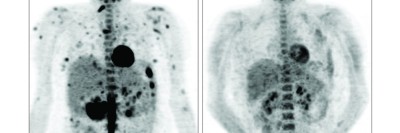
Today, more than a year and a half after starting the drug, Robin’s scans continue to show a significant reduction in her disease. “Nearly all the patients who have stayed on the treatment have enjoyed a lasting improvement in their disease, without progression or recurrence,” Dr. Diamond says.
Helping More People With Histiocytosis
The FDA approval of cobimetinib for histiocytosis represents many years of investigation by MSK researchers. It builds upon the landmark approval of vemurafenib (Zelboraf®) in 2017 for the treatment of Erdheim-Chester disease. That drug, like cobimetinib, was originally developed to treat melanoma. Vemurafenib targets a mutation called BRAF V600E, which is found in about 50% of histiocytosis cases.
“We were looking for ways to help the 50% of patients who did not have BRAF V600E,” says MSK hematologic oncologist Omar Abdel-Wahab, MD.
Research in Dr. Abel-Wahab’s lab first uncovered the link between MEK and histiocytosis. Analysis from MSK-IMPACT®, MSK’s tumor sequencing test, revealed that more than 90% of all histiocytosis patients had a MEK gene mutation.
“That testing was really essential in identifying the genetic driver of these rare diseases,” he adds.
In 2019, Drs. Diamond and Abdel-Wahab and their colleagues published their findings in Nature. That study led to the FDA granting Breakthrough Therapy Designation for the use of cobimetinib in treating histiocytosis.
“Remarkably, this drug appears to work even in patients who don’t have the MEK mutation, suggesting that the drug may have broader effects,” says Dr. Abdel-Wahab, Director of the Center for Hematologic Malignancies and Chair of the Molecular Pharmacology Program at the Sloan Kettering Institute.
Advancing Treatments for Both Children and Adults With Histiocytosis
Histiocytosis affects about 2,000 people in the United States every year. MSK’s pediatric oncology program, MSK Kids, has a team of experts in treating children with the disease, led by pediatric oncologist Maria Luisa Sulis, MD, MS.
“Looking ahead, we are working on advancing treatment options for pediatric patients with histiocytosis,” Dr. Abdel-Wahab says. MSK is planning clinical trials to evaluate cobimetinib in younger patients.
Cobimetinib Allows Robin To Feel Like Herself Again
When Robin first started taking cobimetinib, she experienced some side effects, including extreme fatigue and anxiety. Once Dr. Diamond reduced her dose, she started feeling better right away. Over a few months, the buildup of histiocytes in her body greatly decreased. Her only current side effect from the cobimetinib is mild leg pain, which is treatable.
Robin and her husband, Gregory, travel to MSK from Baltimore every three months for her checkups. Robin is grateful that she is able to get her scans and see most of her care team — which also includes ophthalmic oncologist Jasmine Francis, MD — in one place: the David H. Koch Center for Cancer Care at MSK.
When she’s not at work, Robin, now 63, enjoys spending time with her family, especially her 2-year-old granddaughter. She’s also begun taking kickboxing and water aerobics classes.
“Prior to taking cobimetinib, I didn’t have any energy for a long time,” Robin says. “Dr. Diamond really encouraged me to get out there and start doing things. I’m finally feeling like myself again.”