Drug development is a complicated task. In the era of targeted therapies for cancer, medicinal chemists seek to design drugs that selectively block the activity of proteins driving tumor growth. But molecules that block these cancer-causing proteins don’t always make effective drugs. Now, for one such protein, called KRas, a collaborative team from Memorial Sloan Kettering and Harvard University is reporting the first step of an approach to make targeted drugs that are more effective.
KRas acts as a switch for driving cellular growth and survival. Because mutations in KRas are linked to nearly 30% of all cancers, the hunt for molecules that can regulate its activity is a critical area of focus. But KRas has long been thought of as an “undruggable” target, in large part because it binds so tightly to its natural partner, a molecule called GTP. This has made it difficult to find drugs that can block that binding using traditional drug-discovery techniques.
“The most promising molecules for blocking KRas are small proteins called peptides, but these are not great options because the body’s metabolism breaks them down,” says Andrew Roberts, a postdoctoral fellow in the Sloan Kettering Institute’s Chemical Biology Program and one of the authors of the new study. “We want these peptides to be drugs, but they’re not ideally suited.”
Mirror, Mirror in the Lab
Now researchers in the laboratory of chemist Samuel Danishefsky at MSK may have found an approach to make such peptides stable and less susceptible to being broken down. The insights come from the area of stereochemistry. Many molecules have left or right “handedness,” meaning that they cannot be superimposed on their mirror images. (To visualize this, picture a glove made to fit on a right hand. It doesn’t matter which way you rotate it; it will never fit properly on the left hand.)
Proteins naturally created in the body are said to have L stereochemistry (L stands for laevus, the Latin word for “left”). Their mirror images, which are made in the lab, have D stereochemistry (for dexter, the Latin word for “right”). Accordingly, the body’s metabolism is better suited to break down proteins and peptides that are left-handed, not right-handed, because the body itself creates them.
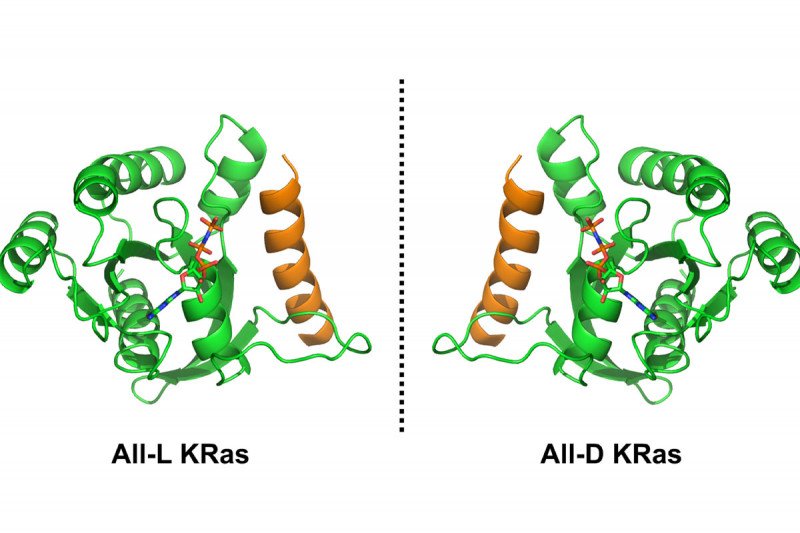
The researchers are exploiting this principle using a technique called mirror-image yeast display, aimed at discovering mirror-image peptides that can block KRas but evade the body’s natural metabolism. To start, they created an unnatural D version of the KRas protein by building it chemically in the laboratory.
This required a major effort in chemical synthesis, leveraging approaches developed by the Danishefsky lab over the past decade. In collaboration with Harvard professor Gregory Verdine and postdoctoral fellow John McGee, the researchers showed that this unnatural protein can fold properly and that it’s able to bind a mirror-image analogue of GTP.
Access to this mirror-image version of KRas will now allow the team to screen a large library of L-peptides and determine which ones do the best job of blocking D-KRas. Once they identify the best candidates, they can synthesize the mirror-image D versions of these peptides, which should be active against natural L-KRas. And because these D-peptides are mirror images of those that occur naturally, they should not be recognized by the body’s metabolism, which would otherwise break them down. These D-peptides should be valuable starting points for developing new drugs to block KRas.
“This is an approach to drug discovery that’s been around for several years, but it’s the first time that anyone has tried it with KRas,” explains Adam Levinson, the first author of the study and a recent graduate of the Tri-Institutional PhD Program in Chemical Biology. “Our lab is very good at synthesizing proteins and other large molecules, and we thought this project would be a perfect application of our expertise to this important cancer target.”
A Central Contribution from a Giant in the Field
This research, published recently in the Journal of the American Chemical Society, was led by Dr. Danishefsky, an eminent chemist who recently retired after a more than 50-year career, including 25 years at MSK.
A leader in the field, Dr. Danishefsky is widely known for his research into natural-products chemistry and his ability to synthesize large, complicated molecules in the laboratory that have contributed to a number of treatment approaches in oncology.
“This research is remarkable and is likely to have significant impact on the field,” says SKI Director Joan Massagué. “It’s an important contribution from a giant of synthetic chemistry.”