Positron emission tomography (PET) is one of the most widely used diagnostic tools in cancer care. PET scans show the location of a tumor, usually based on increased glucose intake, a hallmark of cancer cells.
Despite their effectiveness, PET scans can be hard to access in developing countries and rural areas of the U.S. and other developed countries. PET scanners are expensive — about $1.5 million — and many regions lack the infrastructure required to house them and support their operation. There has been a pressing need for cheaper ways to do imaging to improve cancer care for a much larger population around the world.
Now a team of researchers led by Jan Grimm, a radiologist and molecular imaging specialist at Memorial Sloan Kettering Cancer Center (MSK), has begun testing a new technology that could help solve this problem. The technique involves employing specialized cameras to detect Cerenkov light, a faint glow that is naturally emitted by radioactive tracers. PET scans require these radioactive tracers to detect the increased glucose metabolism of cancer cells.
The new approach could make it possible to get critical information with most clinically used radioactive tracers but without the PET machine — and at a fraction of the cost.
“This imaging technique based on Cerenkov light could be used instead of expensive PET scans in many cases,” Dr. Grimm says. “It wouldn’t replace PET because it cannot provide the same level of detail, precise measurement, or depth penetration. But having a cheaper and faster imaging approach to select patients that should undergo a PET scan or as a first-line test could provide a huge benefit to many developing areas.”
The researchers demonstrated the effectiveness of Cerenkov light in this setting in a clinical trial of nearly 100 patients. For five different radiotracers, Cerenkov proved to be as accurate for showing tumor location as conventional imaging methods like PET and computed tomography (CT). For some patients who were receiving radiation therapy, Cerenkov also provided information on whether their tumors were shrinking or responding in other ways to the treatment.
The researchers reported these results on April 11 in Nature Biomedical Engineering.
A ‘Sonic Boom’ for Light
Cerenkov light is given off when charged particles (electrons or positrons) travel through a medium, such as water or tissue, faster than light can travel through that same medium. It has been described as kind of visual “sonic boom.” Although Cerenkov light was discovered more than 100 years ago, it has only recently been considered for biomedical imaging purposes.
Over the past decade, Dr. Grimm’s lab has been investigating various applications for Cerenkov light. In 2013, they showed that Cerenkov imaging could potentially be used to provide information about a tumor’s molecular activity. One major hurdle, however, has been that the light emitted is so low that it required very sensitive cameras — and very dark surroundings — to detect the signal. This meant sealing off light coming from electronics in the room and switching off lights even outside the room.
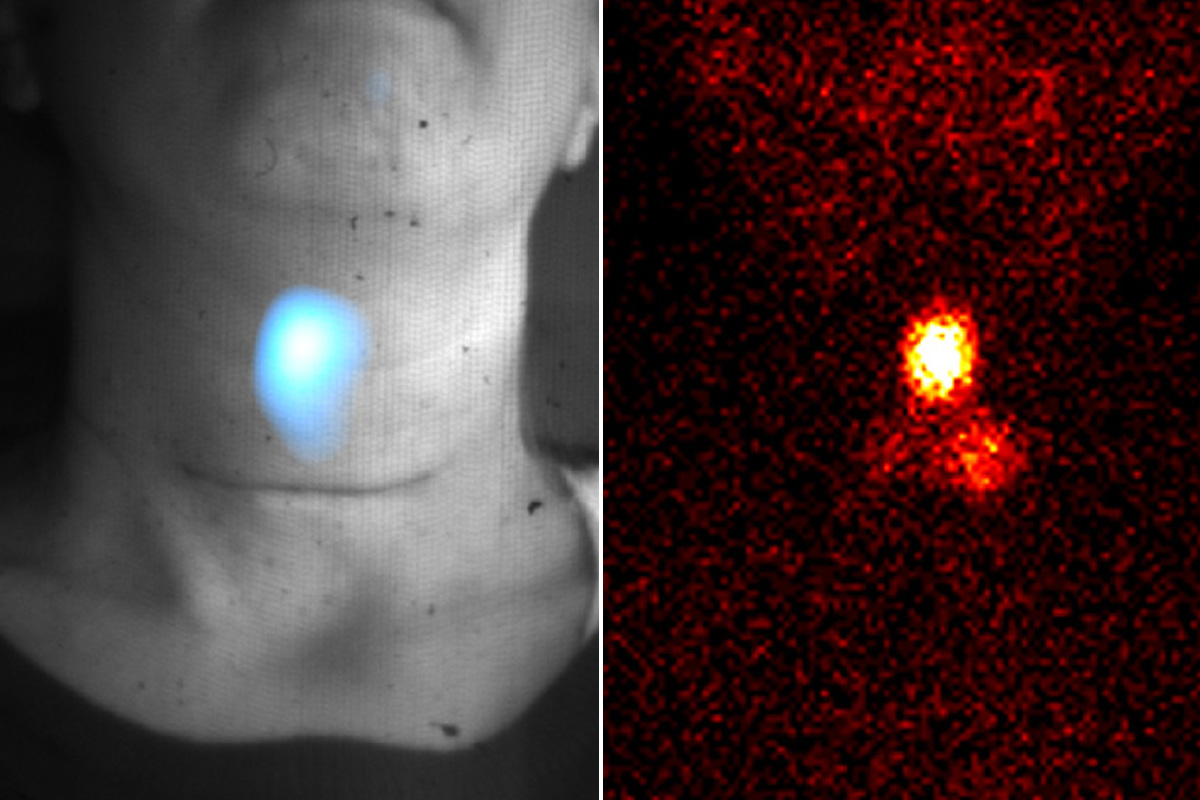
In recent years, a team of researchers in the Sloan Kettering Institute’s Oncologic Molecular Imaging Laboratory of Dr. Grimm have worked on solving this problem. This group includes Edwin Pratt (now of the Jason Lewis Lab in SKI), Magdalena Skubal, and Benedict McLarney of the Grimm Lab, and Pamela Causa-Andrieu of the Department of Radiology.
Funded by a grant from the National Cancer Institute, the team developed a portable device made up of a fiberscope connected to a camera and an enclosure that blocks all light. The enclosure is essentially a black box with three regular sides and a curtain on the fourth side that can be pulled shut.
The patients sit comfortably in a chair inside the enclosure for about 10 minutes. The only light is the Cerenkov light coming from the radiotracer inside their bodies. The camera detects the light, which is too weak for the naked eye, via a fiber leading from inside the box to the camera outside.
“This setup could be wheeled in wherever needed to do imaging, making it useful in many places that can’t have PET imaging readily available,” Dr. Pratt says. “It could offer screening away from a hospital as a first-line imaging test. And then, if something odd is seen that needs closer examination, you can redirect the patient to a comprehensive cancer center or similar place for a PET scan.”
Largest Cerenkov Light Imaging Trial to Date
In the clinical trial, the participants were already receiving radiotracers for more conventional imaging methods as part of their clinical care at MSK, and they kindly agreed to also be imaged using the Cerenkov light method.
“MSK has such a large number of patients getting PET scans or targeted radiotherapy that it made it very easy for us recruit enough people for our study,” Dr. Grimm says. “From a patient’s perspective, they were just agreeing to let us take an extra set of images after they received the radiotracer.”
The clinical trial was the largest study to date testing Cerenkov imaging. While it validates the technique’s potential as a cheaper and more portable imaging approach than PET, Dr. Grimm and his colleagues are already looking for further refinements to make it even more useful. This includes imaging the entire body (rather than specific sites), imaging during an operation to help guide surgical decisions, and using a more sensitive camera to make image acquisition even faster and easier.
- PET scans can be hard to access in developing countries and rural areas of developed countries.
- Cerenkov light is a faint glow that is naturally emitted by radioactive tracers used in PET scans.
- Special cameras detecting Cerenkov light can provide some of the basic diagnostic info found with PET scans
- Cerenkov-based imaging could be a cheaper, faster alternative to PET scans in many cases.