
Many of us remember from high school biology class that mitochondria are the cell’s “power plants.” These small kidney-bean-shaped structures are what convert nutrients from food into ATP — the cell’s “energy currency.” Cells spend that currency every time they perform basic cellular activities, whether that’s encoding memories in nerve cells or detoxifying chemicals in liver cells.
This story is true, but it’s incomplete. Cells need more than just energy to live, they also need building blocks — the raw materials from which to make copies of their components so that when they grow and divide, each new cell receives a full and equal share of parts.
For many years, it was not clear where in the cell these building blocks are made. But over the past decade, scientists have learned that mitochondria control this process too. Instead of using nutrients to make ATP, mitochondria can use them to make the cellular building blocks that will form DNA, new proteins, and new cell membranes.
How do mitochondria choose which of these two opposing paths to take?
“That was the question we set out to answer,” says Craig Thompson, MD, a member of the Cancer Biology and Genetics Program in the Sloan Kettering Institute at Memorial Sloan Kettering Cancer Center (MSK) and the senior author of a new paper published November 6, in Nature. “How do mitochondria balance these two essential functions that they do for all cells in our body?”
Under typical circumstances, he says, it’s easy for cells to square their balance sheets. When nutrients are plentiful — when our cells are getting all the nutrients they need and then some — cells can use those nutrients to make an adequate supply of ATP and also to make enough cellular building blocks for growth and division.
But what happens during times of stress, when nutrients are scarce and demand for both ATP and cellular building blocks is high? No one knew the answer to that question.
How Cells Survive Under Stress
To appreciate the dilemma a cell faces, Dr. Thompson says, consider what happens when you cut yourself.
“The blood starts to pour out, and with it the nutrients that normally sustain the tissue. The cells are now in a stressful situation. They urgently need ATP to spend on the healing process and they also urgently need new supplies to repair the wounded tissue. How the cell decides between these competing demands hasn’t been clear.”
In their new paper, Dr. Thompson and his colleagues show in exquisite detail how mitochondria tackle this vexing problem. Through a dramatic and dynamic process of physical and chemical transformation, mitochondria form distinct subpopulations that are specialized for satisfying each of the competing demands.
The end result is an almost perfect division of labor, with one subpopulation outfitted with the machinery for making ATP, and one subpopulation outfitted with the machinery for building new cell structures.
The new findings not only answer a fundamental question about cell biology, they have direct implications for understanding cancer — the epitome of a stressful biological event.
An Apple Pie Analogy
Dr. Thompson and his colleagues, led by Keunwoo Ryu, PhD, a postdoctoral fellow in the lab, started by asking what would happen if they put cells in a stressful situation, where, for example, there is a low amount of the nutrient glucose and simultaneously a high demand for ATP. You might suspect that the cells would favor making ATP at the expense of making cellular building blocks. That is not, however, what the researchers found.
“The increased demand for ATP didn’t in any way compromise the cells’ ability to make other molecules for growth,” Dr. Thompson says.
That was a very odd finding, one that seemed to “break the laws of thermodynamics,” he adds.
It would be as if a baker started with the ingredients to make one 12-inch apple pie but at the end of cooking, had two 12-inch pies. That told the scientists that something very unusual was going on.
New Research Shows That the P5CS Enzyme Controls the Mitochondrial Division of Labor
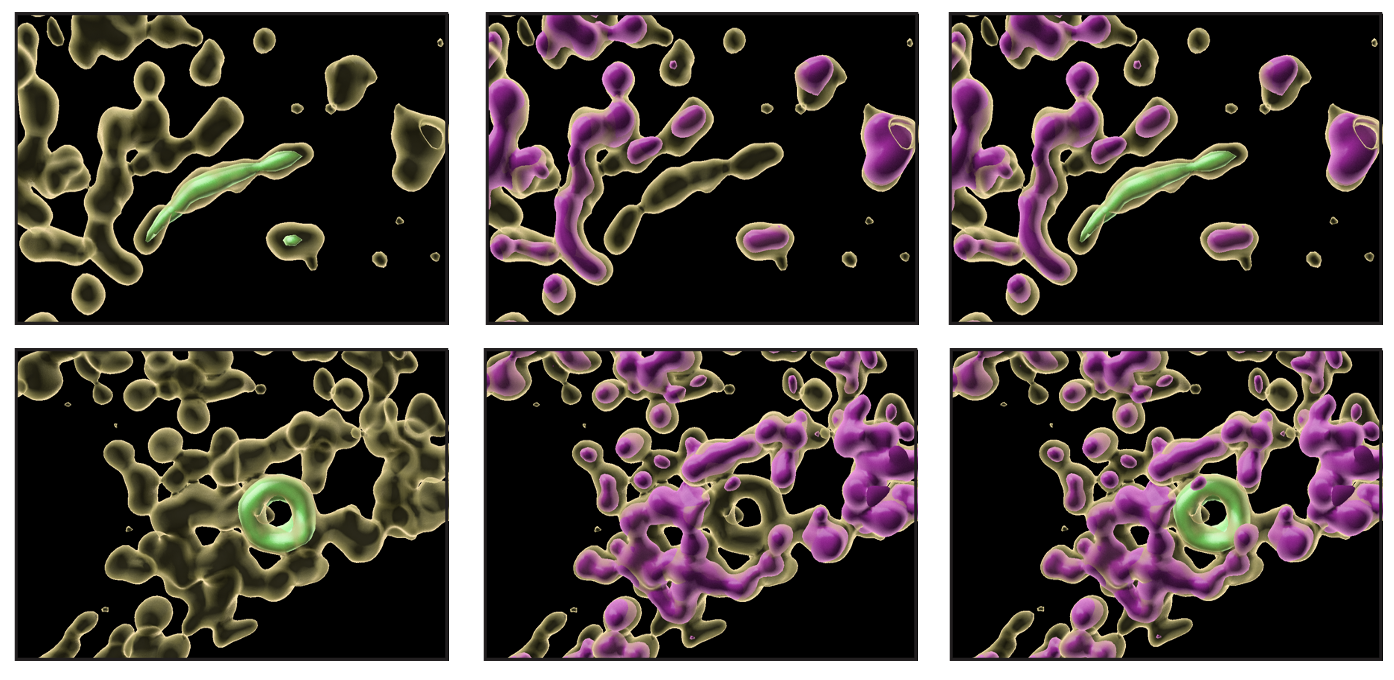
One clue to the mystery of how mitochondria can perform two functions at once came from looking at which enzymes the two different pathways have in common. They found only one: an enzyme called P5CS.
“P5CS is a kind of linchpin protein that is necessary to make the judgment between these two pathways,” Dr. Thompson explains.
When the team looked in more detail at what P5CS was doing in the stressed-out cells, they saw that individual P5CS enzymes had joined together to make long filaments. But curiously, the filaments formed in only one subpopulation of mitochondria; in the other, they were absent.
The subpopulation of mitochondria with the P5CS filaments were noticeably different in other ways. Typically, in mitochondria that can make ATP, the inner membrane of the mitochondria forms intricate folded structures called cristae, which are often visible in the mitochondria shown in textbooks. But in the mitochondria rich with P5CS, the cristae were absent.
Upon further probing, it became clear that the two subpopulations had completely segregated their roles, with one population becoming streamlined for just making ATP and one population becoming specialized for making new cellular building blocks.
An essential upshot of this division of labor is that each subpopulation got better at doing its job, which helps explain why those original stressed-out cells were able to make both enough ATP and enough building blocks to survive and grow in the stressful conditions.
Surprise Finding About Mitochondrial Fission and Fusion
But how do the two distinct subpopulations come about in the first place? Here’s where the story takes another surprising turn. Scientists have known for decades that mitochondria are highly dynamic organelles. They go through fusion and fission events, in which individual mitochondria join together and then split apart, over and over again.
Scientists have hypothesized that the fusion and fission events are necessary to recycle the components of mitochondria damaged from the highly demanding process of ATP generation. That may be true. But this new study shows that the fusion and fission process is also required to segregate the filaments of P5CS into one subpopulation and the ATP-making machinery into the other.
“That was a surprise,” Dr. Thompson says. “I believe this is the first time anyone has shown that mitochondrial fusion and fission are necessary to separate functions of mitochondria into subpopulations.”
How This Mitochondria Discovery Relates to Cancer Cells
Why is this relevant to cancer? Well, as anyone who works in the field knows, cancer cells are able to survive in stressful conditions that typically kill normal cells. For example, cancer cells can survive in the very center of the tumor where nutrients and oxygen are scarce. No ordinary cell can do that.
To see if the mitochondrial changes were happening in the context of cancer, Dr. Thompson and his colleagues looked at tissue samples of pancreatic cancer, one of the most aggressive cancers. Sure enough, the tumors had developed the discrete subpopulations of mitochondria, while the surrounding normal tissue had not.
“These mitochondrial changes seem to be driving cancer progression, at least in pancreatic ductal adenocarcinoma,” Dr. Thompson says. His team is now looking to see if this discovery holds for other types of cancers, as well.
They also want to investigate just how these mitochondrial changes might underlie cancer progression. “It could be that they fuel how cancer cells acquire the ability to metastasize, or spread” he says.
There’s even a possible connection to aging. “We think that understanding these mitochondrial dynamics will be critical for our understanding of how we might facilitate tissue repair and tissue regeneration as we age,” says Dr. Thompson. “When we see these mitochondrial changes, is that a sign that a tissue is under stress? We’re exploring that idea as well.”
Funding
This study was supported financially by the Hunter Douglas Fellowship in Breast Cancer Research, the BRIA Postdoctoral Researcher Innovation Grant, and the National Cancer Institute (grants R35 CA263816, P30 CA008748 and R35 CA283988).
Read the study: “Cellular ATP demand creates metabolically distinct subpopulations of mitochondria,” Nature. DOI: 10.1038/s41586-024-08146-w