
Thanks to a clinical trial (research study) of a targeted immunotherapy drug called mosunetuzumab (Lunsumio™), Anne Schmiedel is now in remission from follicular lymphoma.
“These days I feel really good,” Anne says. “My energy levels are good, and I’m able to do everything I want to do. I’m thankful like you can’t even believe.”
Nearly all the patients in that phase 2 trial experienced some tumor shrinkage, and three-quarters now have no evidence of disease.
These early but promising results were reported by Memorial Sloan Kettering Cancer Center (MSK) lymphoma specialist Lorenzo Falchi, MD, at the 2023 American Society of Hematology (ASH) Annual Meeting.
“We have been waiting for a drug that could replace chemotherapy in these patients,” Dr. Falchi says. “Although we need to follow the patients for a longer period of time, this treatment may be what we’ve been looking for.”
Mosunetuzumab for Follicular Lymphoma: Clinical Trial Results
The trial enrolled people with a type of non-Hodgkin lymphoma called follicular lymphoma that was severe enough to require treatment and who had not previously received any treatment for their cancer. Follicular lymphoma is the most common type of low-grade lymphoma.
Mosunetuzumab was offered to patients instead of standard therapy — which is a combination of chemotherapy and immunotherapy. This approach allowed the participants to avoid common side effects of traditional chemotherapy.
The analysis is based on the first 45 patients to participate in the study:
- 96% had at least some shrinkage of their follicular lymphoma tumors.
- 76% had a complete response, meaning no evidence of disease remained.
How Does Mosunetuzumab Shrink Lymphoma Tumors?
The drug that Anne received, mosunetuzumab, is a bispecific antibody. This novel class of drugs consists of two parts: One part recognizes a marker on the patient’s immune T cells, and the other part recognizes a marker on the patient’s cancerous B cells. The drug acts like a lasso bringing the two cell types together, which allows the patient’s own immune system to destroy the lymphoma.
The Inspiration for Studying Mosunetuzumab for Follicular Lymphoma
“Mosunetuzumab is already approved for treating people with lymphoma whose disease does not respond to standard treatments,” Dr. Falchi says. “Studies have shown that in 80% of those patients, their tumors were partially reduced, and in 60%, the tumors were completely gone.” In these earlier studies, the drug kept the cancer under control for an average of two years.

Dr. Falchi and his colleagues had a hunch: If mosunetuzumab worked in patients who hadn’t received any previous treatment, perhaps they could avoid chemotherapy altogether.
“Chemotherapy has a number of side effects, including a risk of additional cancers,” Dr. Falchi says. “In addition, between 15% and 20% of patients do not adequately respond to it.”
Relief Seeing Cancer Disappear From Scans, Thanks to MSK Clinical Trial
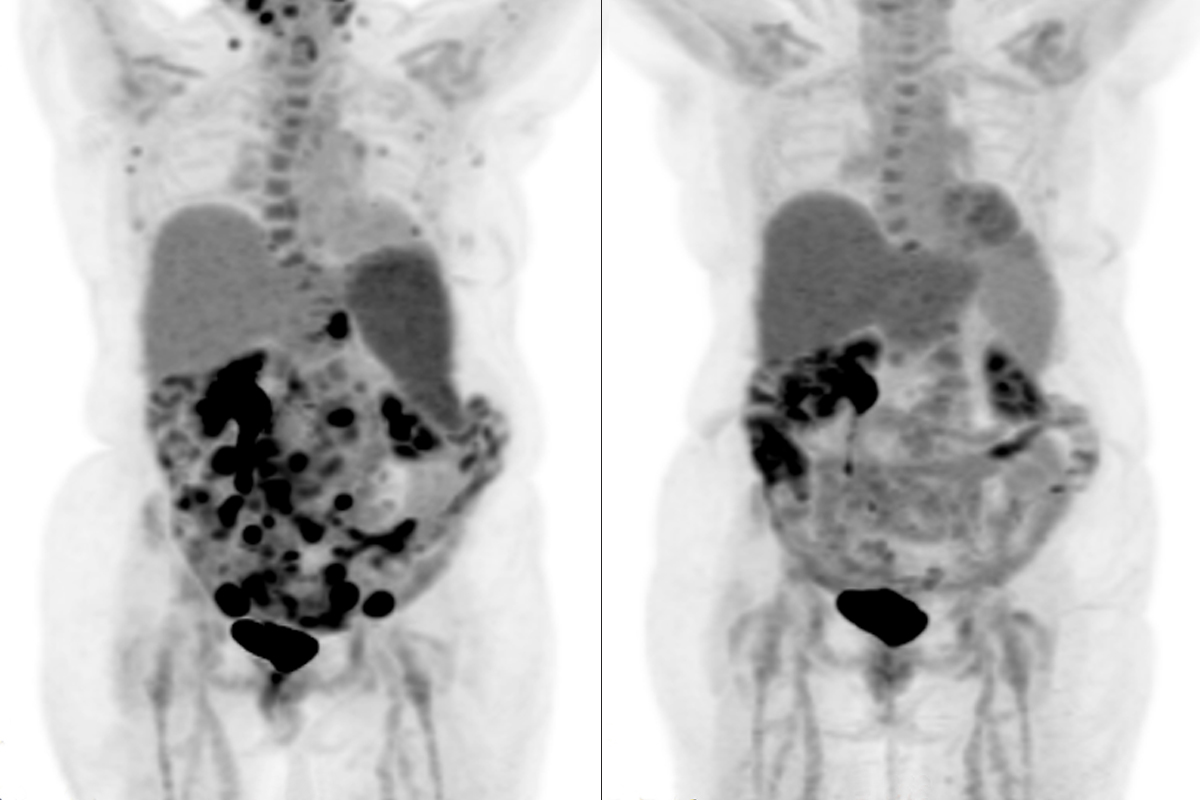
Anne received 10 doses of mosunetuzumab, which is given every three weeks as an injection in the belly, starting in March 2023. When she had her first scan less than three months after beginning the treatments, she was astonished: The cancer was going into remission.
“I still remember the day I saw those scans,” Anne says. “And then in September 2023, I was told I’d had a complete response. It doesn’t get any better than hearing those words.”
About half of people in the mosunetuzumab trial experienced a side effect called cytokine release syndrome (CRS), which can cause fevers, dizziness, and body aches, among other symptoms. It’s a common side effect of drugs that activate the immune system, like mosunetuzumab. Anne experienced CRS just once, after her first dose. Later, a subsequent dose had to be delayed due to low blood counts, but overall, she felt well throughout her treatment.
Anne’s Follicular Lymphoma Treatment at MSK
Anne was first diagnosed in the fall of 2019, after an MRI she received in preparation for spine surgery revealed enlarged lymph nodes in her abdomen, a common symptom of follicular lymphoma. She immediately went to MSK, where she was cared for by lymphoma specialist Paul Hamlin, MD, a co-author of the study presented at ASH.
Dr. Hamlin told her that even though she had cancer, she didn’t need treatment right away. So Anne had the spine surgery, which was successful. Dr. Hamlin and his team continued to monitor her lymphoma regularly with scans and blood tests. This approach is called active surveillance.
About three years later, in the summer of 2022, blood work revealed that Anne had become severely anemic, another symptom of follicular lymphoma. After tests ruled out other possible causes of the anemia, Anne got the news she was hoping not to hear: It was time to begin treatment.
Deciding To Participate in an MSK Clinical Trial and Avoid Chemotherapy
Thankfully, Dr. Hamlin also had some good news. There was a trial that would allow her to avoid chemotherapy.
“I knew there would be risks to participating in a trial, but Dr. Hamlin thought that someone in my situation could really benefit,” Anne says. She remembers that during discussions about what to do, someone in her family said, “MSK is on the cutting edge of everything. If you have the opportunity to receive the latest treatment, why wouldn’t you go that route?”
Anne received most of her treatments at the David H. Koch Center for Cancer Care at MSK in Manhattan, but she has been able to go for some of her blood work and scans at MSK Westchester, closer to her home.
“There’s no way I would have gone anywhere else but MSK,” says Anne, now 70.
Dr. Falchi plans to continue studying the effectiveness of mosunetuzumab for patients who have not yet received other treatments.