
At Memorial Sloan Kettering, scientists, physicians, and administrators strive to develop and commercialize scientific and medical inventions — endeavors aimed at making more-effective and affordable cancer treatments available to patients around the world.
Head and neck surgeon Snehal G. Patel and two of his colleagues are patenting an invention: a device they hope could help advance the field of endoscopic laser surgery, and one day expand treatment options for patients with tumors growing in the throat and other parts of the body.
The device prototype is still in its infancy, and its translation into the clinic might be years away. Yet Dr. Patel calls the invention one of the biggest thrills of his career.
“The vision of creating something that potentially could become available at hospitals everywhere is just tremendous,” he says. “It’s hard to believe something like that could actually happen.”

In the past, something “like that” could almost never happen with academic investigators such as Dr. Patel and his coworkers. But since 1980, researchers at Memorial Sloan Kettering and other nonprofit research institutions have increasingly been able to develop and commercialize inventions and see their brainchildren give rise to practice-changing medical products. That year the US Congress passed the Bayh-Dole Act, which allowed academic institutions to take ownership of discoveries emerging from government-sponsored research and to enter licensing agreements with commercial partners.
“The legislation changed the world by creating an incentive for both academic institutions and investors to engage in technology transfer,” the process by which intellectual property is handed over from the public to the private sector, explains Andrew D. Maslow, who directs Memorial Sloan Kettering’s Office of Technology Development.
That incentive has yielded a steady stream of lifesaving therapies and other practical applications. According to a Boston University study published in The New England Journal of Medicine in February, universities and other nonprofit institutions have played an increasing role in the discovery of new drugs and vaccines during the past four decades. The study also found that, on average, therapies stemming from academic research tend to be more effective than those discovered by the pharmaceutical industry.
In addition, the study examined the individual contributions made by 75 public-sector biomedical research institutions across the country. With nine products approved for marketing by the US Food and Drug Administration, Memorial Sloan Kettering ranks third, surpassed only by the National Institutes of Health and the ten-campus University of California system. (Read about the nine therapies, and other products developed by Memorial Sloan Kettering researchers that now are on the market.)
Thomas J. Kelly, Director of the Sloan Kettering Institute, attributes this extraordinary track record to a unique academic culture, fostered at Memorial Sloan Kettering over several decades, with basic, translational, and clinical scientists working side by side.
“We place a very high value on the translation of new knowledge into practical applications that address medical needs, and have invested in a first-rate research infrastructure in pursuit of this mission,” he says.
But the most promising applications of cancer research are yet to be reaped, Dr. Kelly reflects. “Only in recent years have we learned enough about the genes and biological processes that drive the growth and progression of cancers to begin to develop therapies that specifically target those processes,” he says. “There’s never been a better time to accelerate the development of new therapies and medical innovations for the benefit of patients.”
From Bench to Business
The Office of Technology Development helps Memorial Sloan Kettering’s researchers identify discoveries that potentially could be developed into clinical applications — such as drugs or medical devices — and seeks patents for such inventions to safeguard their exploitation for the common good.
As soon as a patent application has been filed, Memorial Sloan Kettering licensing managers look for ways to move the research forward, toward manufacturing, human trials, and FDA approval — which may cost hundreds of millions of dollars — for example, by licensing patents to an established biotechnology or pharmaceutical company, or by forming a start-up company.
“Creating patents and commercial products isn’t the essential goal of research here,” notes Sharon R. Seiler, who directs the technology management and commercialization unit in the Office of Technology Development. “The endeavors of our faculty span a wide spectrum of science — and every so often, commercially valuable discoveries do emerge.” In the past two years, she and her colleagues have helped the faculty secure more than 115 patents and negotiate about 70 licensing deals.
“In handing such discoveries over to our corporate partners, our goal is to move effective therapies into the clinic as swiftly as possible so that patients might benefit from them,” says Eric M. Cottington, who oversees Memorial Sloan Kettering’s technology transfer activities as Vice President for Research and Technology Management.
In addition, technology transfer brings in revenue from licensing fees and royalties. “Part of these returns is distributed to the inventors and the remainder is pumped back into the Center’s research budget, fueling new discovery,” Dr. Cottington notes.
The office also hosts a busy agenda of activities to educate Memorial Sloan Kettering’s faculty on a broad range of topics — from the basics of patenting to the emotional aspects of starting a business. “More and more of our faculty are looking for ways to be actively involved in the commercialization and future development of their inventions,” says Jill Fraser, who manages marketing, communications, and strategic initiatives in the Office of Technology Development.
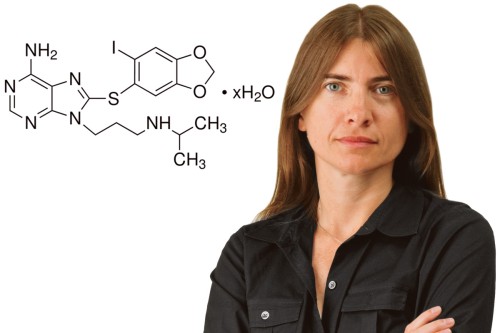
One of these investigators is chemical biologist Gabriela Chiosis, of Sloan Kettering Institute’s Molecular Pharmacology and Chemistry Program. Together with a coworker, she recently launched a small biotech company with funding from a private foundation. The company, Samus Therapeutics, LLC, has licensed patents pertaining to PU-H71, a novel targeted therapy discovered in Dr. Chiosis’s laboratory. The drug holds promise for treatment of several cancer types.
“We chose a start-up model rather than trying to license our patents directly to a pharmaceutical company, because it gives us the opportunity to be involved in the early stages of clinical development, which are now under way,” Dr. Chiosis says. “By taking experiences from the clinic back to our laboratory, we hope to push forward with our science as far as we can to ensure that our work will generate true value for patients.”
Samus is one of four businesses that has been spun out of Memorial Sloan Kettering research in the past couple of years. “But commercial applications of therapeutic discoveries and tech transfer tend to be secondary interests for our faculty,” observes David A. Scheinberg, who chairs Sloan Kettering Institute’s Molecular Pharmacology and Chemistry Program. “Our primary goal is the discovery of new knowledge.”
Equipped for Effective Translation
Sometimes, the discovery of that new knowledge illuminates a path to medical innovation — such as a potential new drug or diagnostic tool. But to effectively translate such knowledge from lab to clinic, scientists must often navigate a funding vacuum, known in industry as “the Valley of Death” — a place where inventions can stall for years or die on the vine, Dr. Cottington explains.
“When the exploration of a new therapeutic concept reaches a certain stage, investigators are less likely to obtain government funding for their research,” he says. “Yet at that point, private companies and venture capitalists are often too risk averse to invest in the development of a new product.”
Memorial Sloan Kettering is making significant efforts to help investigators maneuver new discoveries across the treacherous abyss. For example, Center investigators have access to 36 state-of-the-art core facilities providing a wide range of expert services and research technology. A number of these core facilities are specifically focused on therapeutic discovery, development, and manufacturing, which enables investigators to advance medical innovations in-house — “as far as is needed to encourage commercial entities to step in,” Dr. Cottington says.
In taking advantage of these internal resources, Mark G. Frattini, a physician-scientist who splits his time between doing laboratory research and caring for patients with blood cancers, has been able to turn a scientific notion into a prospective drug.
Since his college years, Dr. Frattini has been studying the biological processes that allow cells to replicate their DNA and divide. “I imagined that by targeting a vital pathway that controls DNA replication, it might be possible to kill cancer cells more selectively,” he says. Six years ago, he and his colleagues began focusing on a protein called Cdc7, which he believes is an ideal target for this type of therapy, “an Achilles’ heel on cancer cells,” he explains.

With help from Memorial Sloan Kettering’s core facility for high-throughput screening, Dr. Frattini and his coworkers sifted through more than 300,000 chemical compounds, searching for a molecule that could be used to block the activity of Cdc7 in tumor cells. Their best find, a naturally occurring compound called MSK-777, was further investigated and developed together with core facility staff specializing in antitumor assessment, genomics, and analytical pharmacology.
“MSK-777 is an entirely homegrown drug candidate,” Dr. Frattini concludes. “Our research in cancer cell lines and mouse tumor models suggests that it might have the potential to destroy tumor cells in patients whose cancer is resistant to many currently used drugs.”
The Experimental Therapeutics Center (ETC), one of Memorial Sloan Kettering’s specialized collaborative research initiatives, partially funded these investigations, as well as those of Dr. Chiosis. Established with a generous donation from the Commonwealth Foundation, the ETC brings together clinicians and scientists throughout Memorial Sloan Kettering who are exploring innovative ways to treat all forms of cancer, supporting their research from concept through early-stage clinical trials. In addition, the ETC funds some of Memorial Sloan Kettering’s core facilities and hosts discussion forums focused on drug discovery and development.
Ongoing projects funded by the ETC include eight preclinical investigations and 11 early-stage clinical trials of experimental cancer therapies — an eclectic pipeline featuring small-molecule drugs, therapeutic antibodies, and gene therapy, as well as vaccines and other immune-based treatments.
“We’re exploring a broad range of therapeutic strategies because ultimately we don’t know what’s going to work,” says Dr. Scheinberg, who directs the ETC. “Our goal is to make long-term investments in scientifically groundbreaking concepts, which often means a concept might be years away from being tested in the clinic when we start funding it.”
For example, with support from the ETC, Memorial Sloan Kettering researchers have paved the way for cell-based cancer therapy in which a patient’s own T cells are genetically engineered to seek out and kill tumor cells. Today the strategy holds promise for the treatment of leukemia and other cancers. But when the research began, a decade ago, not many people would have thought engineered cells could be used as cancer therapy, Dr. Scheinberg observes. (Read more about the research on page 11.)
The Technology Development Fund is another Memorial Sloan Kettering-wide resource that helps investigators turn basic discoveries into commercially potent products. Established in 2009, the fund hands out awards of up to $500,000 to kick-start the development of new therapies and technologies. The committee that reviews proposals includes prominent venture capital investors and business executives. “Proposals get vetted by people who have extensive experience assessing the commercial potential of new discoveries,” Dr. Cottington says.
To date, the fund has financed the development of five drugs and three medical devices. One of these products-to-be, a therapeutic cancer vaccine, was recently licensed to a biotech company called Formula Pharmaceuticals.
Developed in Dr. Scheinberg’s laboratory, the investigational vaccine is designed to incite the immune system’s inherent ability to defend the body against foreign intruders. It is made out of small pieces of a protein called WT1, which is produced by many solid tumors and blood cancer cells, but not usually found in normal cells.
“If you could train the immune system to recognize and attack cells that have WT1 using vaccination, it might be possible to destroy tumors while doing minimal harm to healthy cells and tissues,” Dr. Scheinberg explains. Findings from the first clinical studies of the vaccine done at Memorial Sloan Kettering indicate that this strategy bodes well for the treatment of two aggressive cancer types, acute myeloid leukemia (AML) and mesothelioma.
The ETC and Technology Development Fund paid for the development and manufacturing of the vaccine in large quantities, enabling an ongoing phase II trial, in which the safety and efficacy of the vaccine will be evaluated in a large group of AML patients. Another phase II trial, funded by the US Department of Defense, is under way in patients with mesothelioma.
Forging New Alliances
Drug development is notoriously expensive, lengthy, and inefficient, Dr. Cottington notes. Memorial Sloan Kettering has recently teamed up with a number of corporate and noncorporate organizations in an effort to speed the process.
For example, Memorial Sloan Kettering recently joined Pfizer Inc.’s Centers for Therapeutic Innovation (CTI) along with six other research institutions in New York City. Housed in the Alexandria Center for Life Science — a biotech park launched last year on the east side of Manhattan — the partnership is intended to achieve what the company refers to as a new model of drug discovery, in which Pfizer’s resources and technology infrastructure are married with the intellectual resources of academia.
Dr. Cottington believes initiatives such as the CTI are emerging in response to a hardening industry climate. “Drug makers are under huge pressure to invigorate their pipelines, and may increasingly rely on collaboration with academic researchers to stay profitable in the future,” he says.
Medical oncologist Paul B. Chapman, of Memorial Sloan Kettering’s Melanoma and Sarcoma Service, is leading investigations of a therapeutic antibody that targets melanoma tumor cells, soon to be co-developed by Memorial Sloan Kettering and Pfizer. Originally developed and produced at Memorial Sloan Kettering under the leadership of physician-scientist Alan N. Houghton, “the antibody has already undergone extensive clinical testing here at Memorial Sloan Kettering and elsewhere,” Dr. Chapman explains. “Based on these trials, we already know it is effective against melanoma.”
With support from the CTI, he and his colleagues will engineer a new version of the antibody through a process called humanization, which they expect will enhance the effectiveness of the drug. The team will collaborate with Pfizer scientists and experts, and have access to the CTI’s advanced technology for antibody production.
Under the terms of the partnership, Pfizer will fund the development of the humanized antibody throughout preclinical testing and phase I clinical trials. If the therapy continues to show promise after this first phase of development has been completed, Pfizer will develop it further and pay Memorial Sloan Kettering royalties on future sales. In return, the company will have the opportunity to broaden its portfolio with a promising drug candidate.
Disease foundations and other interest groups are also launching initiatives to help scientists deliver more quickly on the clinical promise of new discoveries. For example, Memorial Sloan Kettering is one of four institutions recently selected by the Leukemia & Lymphoma Society (LLS) as academic partners of the LLS’s Therapy Acceleration Program. Dr. Frattini and his colleagues will receive up to $3 million from the LLS to help advance the development of MSK-777 toward early-stage clinical trials in leukemia patients whose disease does not respond to other treatments.
“We’re also getting help with the extensive administrative procedures the FDA requires before clinical trials can start,” Dr. Frattini explains. “The partnership has been amazing in terms of coordinating scientific and administrative efforts so that no time is lost in moving the research from the laboratory to the clinic.”
The Center is also participating in civic initiatives to enhance New York City’s entrepreneurial ecosystem in the area of life sciences — efforts organized by the New York City Investment Fund (NYCIF), the New York City Economic Development Corporation (NYCEDC), and the Partnership for New York City.
For example, the Office of Technology Development hosts Riverside Chats — a monthly seminar series attended by graduate students, faculty, and postdoctoral fellows from across the city’s biomedical research institutions — and biotechnology networking events, on behalf of NYC Tech Connect. Established earlier this year by the NYCIF and the New York City Council, NYC Tech Connect offers programs, expertise, and resources to accelerate the formation of start-up companies around the city’s prominent research institutions.
Two years ago, the NYCIF and NYCEDC launched a citywide competition called the BioAccelerate NYC Prize, which funds biomedical research with the goal to spur local business development and create new jobs. Earlier this year, the prize was awarded to five New York City researchers, who each received a grant of $250,000. In addition, the awardees were paired with mentors — senior industry experts who are helping the investigators develop and commercialize their research projects.
One of these recipients is Memorial Sloan Kettering clinician-scientist Michelle S. Bradbury, who specializes in neuroradiology and nanomedicine. She and her colleagues have developed a silica nanoparticle platform for the targeted detection of cancer cells. The technology has been approved by the FDA for investigation in patients, and its clinical development is supported by Memorial Sloan Kettering’s Technology Development Fund.
“The new particle technology could be used in combination with state-of-the-art optical PET imaging devices to detect cancer cells in the lymph nodes of patients with metastatic melanoma,” Dr. Bradbury explains. She and her colleagues are hoping that the use of these combined technologies could be extended to a variety of cancers, allowing doctors to determine the extent of a cancer’s spread more accurately, and thereby tailor personalized treatment with better precision. Jerry Korten, the former CEO of Versamed, is Dr. Bradbury’s industry mentor for the BioAccelerate award.
“Our regional policy makers are actively promoting academic research in areas such as biomedicine and engineering, venturing to grow a robust biotech industry similar to that of the San Francisco Bay Area or Boston,” Dr. Cottington notes. Traditionally, scientific discoveries made in New York City have often been commercialized in other states.
“That’s now beginning to change,” he affirms. “We look forward to supporting efforts to grow businesses and jobs here, and to help build a local environment where applied science can thrive.”


