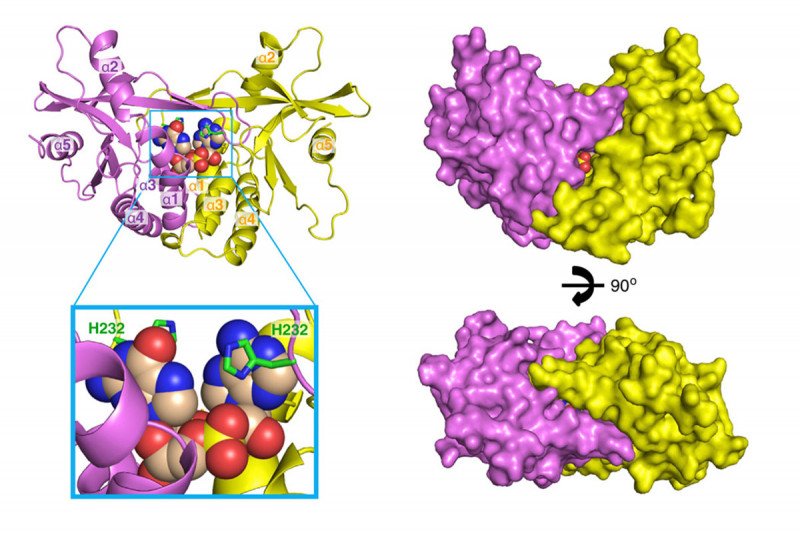
Sloan Kettering Institute structural biologist Dinshaw Patel determined the crystal structure of key parts of the STING pathway. Here, different viewpoints of the structure are shown. Images courtesy of Cell Press.
Drugs called immune checkpoint inhibitors have made a significant difference for some people with cancer. They work by taking the brakes off the immune system, allowing white blood cells called T cells to attack a tumor.
For this approach to work, however, the T cells need to be able to see the tumor and recognize it as something that doesn’t belong in the body. Often, they cannot. That explains why, for most people, these drugs are not effective. Finding new tactics for making tumors more noticeable to the immune system is an important area of research.
Tumors are sometimes described as “hot” if they show signs of inflammation, with lots of immune activity around them. “We’re looking for ways to turn a cold tumor into a hot tumor,” says Memorial Sloan Kettering physician-scientist Liang Deng. “If you can bring the tumor out of hiding and make it more visible, it will help to really ramp up the immune response.”
Finding Ways to Trick Cancer Cells
One approach that many investigators around the world are studying is the potential to harness the cGAS/STING pathway. (The abbreviation cGAS/STING is a much shorter way of saying “cyclic GMP-AMP synthase/stimulator of interferon genes.”)
In particular, cGAS/STING works by detecting bits of DNA from bacteria or viruses that have infected a cell. The detection fires up the innate immune pathway, the system of immune defenses that are present from birth and are always active. Innate immune cells produce chemicals that alert other parts of the immune system to the presence of the intruders. In 2013, Sloan Kettering Institute structural biologist Dinshaw Patel published two papers in Cell showing some of these complex structures for the first time.
Now, some pharmaceutical companies are starting to develop drugs called STING agonists. These are small molecules designed to activate the STING pathway after being injected into a tumor, which sends out a beacon for immune cells to follow. The idea is to use these new drugs in combination with checkpoint inhibitors.
“STING agonists are based on the hypothesis that you can trick immune cells into thinking that the tumor cells are infected with a virus,” says MSK physician-scientist Samuel Bakhoum. “Then the immune cells will come in and basically clear the cancer away.”
Seeing the Full Immune Picture
More recently, however, investigators have learned that in some cases the STING pathway plays a role in helping cancers thrive, making this approach more complicated. “It turns out that many cancer cells also have DNA where it doesn’t belong. Rather than being only inside the nucleus where it normally resides, it’s also floating around inside the cytosol [fluid] of the cell. This is caused by a phenomenon called chromosomal instability — a widespread feature of human cancer,” Dr. Bakhoum says.
“Chromosomally unstable cancer cells have found ways to adapt to that floating DNA. They avoid the harmful consequences of cGAS/STING activation while using this pathway to their advantage,” he adds. “Alternatively, a small number of tumors lose cGAS and STING altogether. This adaptation to DNA in the cytosol may actually help them spread to other parts of the body.” In January 2018, Dr. Bakhoum was the first author of a paper in Nature that reported this phenomenon.
Along with researcher Lewis Cantley of Weill Cornell Medicine, Dr. Bakhoum recently published a review article in Cell on the ways that cells with unstable chromosomes use STING to their advantage to evolve and become more aggressive. It turns out that chronic activation of this pathway might suppress the immune system rather than trigger it to fight the cancer. “It suggests that we need to be very careful in determining which people could benefit from treatment with STING agonists,” Dr. Bakhoum says. “Patient selection will be a critical contributor toward the success of this therapy.”
Another Approach to Heating Up Tumors
Dr. Deng’s lab is taking a different tack for activating innate immunity in tumors: injecting them with a virus. This is another way to flag tumors and make them more visible to the immune system.
She’s working with modified vaccinia virus Ankara (MVA). This engineered virus has been safely used as a vaccine against smallpox. In 2017, her laboratory published a paper in Science Immunology demonstrating that injecting inactivated MVA into tumors in mice stimulates the immune response against the tumors. The findings showed that the response was boosted by checkpoint inhibitor drugs.
Now her laboratory is working on engineering MVA to make it more potent for immunotherapy. Dr. Deng explains that using the engineered MVA has several potential advantages over drugs designed only to fire up STING. For one thing, the virus is larger than a drug molecule, allowing it to remain in the tumor tissue for a longer time. In addition, the virus can be engineered to do much more than draw attention to the tumor.
The engineered MVA activates STING not only in tumors but T cells too. It also carries a growth factor for immune cells called dendritic cells. “We know based on previous work that dendritic cells are an important part of the immune response to cancer,” she says. “Injecting engineered MVA into tumors creates an in situ vaccination effect, which teaches T cells to recognize tumors.”
Dr. Deng and her MSK colleagues Jedd Wolchok, Taha Merghoub, and Stewart Shuman recently co-founded a start-up company called IMVAQ Therapeutics. The company is developing the virus so that an application can be submitted to the US Food and Drug Administration to begin clinical trials. IMVAQ is planning tests in a number of solid tumors, either alone or in combination with checkpoint inhibitors. “We hope this approach will be particularly successful in tumors that don’t usually respond to checkpoint inhibitor drugs, like breast and prostate cancers,” she notes. “We also believe this virus will be very safe because it doesn’t replicate in human cells.”
MVA is not the only virus being studied for this purpose. MSK already has other trials underway that use this immunotherapy approach as well. A phase III trial using a virus called T-VEC (talimogene laherparepvec) is being studied in combination with the checkpoint inhibitor pembrolizumab (Keytruda®) for advanced melanoma, for example.
“All of the research that’s been done over the past 20 years on the basic science of the innate immune system, including a lot of work done at MSK, has made these kinds of studies possible today,” Dr. Deng concludes.