Dave Norkin remembers very clearly the first time he met with MSK physician Diane Reidy-Lagunes. It was his daughter’s birthday. She had just turned 3. Then age 39 and an attorney with the US Navy, Dave had come to see Dr. Reidy-Lagunes after being diagnosed with a gastrointestinal neuroendocrine tumor that had spread to his liver. The doctors at the hospital in Japan, where he was diagnosed, told him that he had — optimistically — just months to live.
But when he saw Dr. Reidy-Lagunes, she saw hope.
“I remember thinking, based on her attitude when I saw her at the time, that there might be more hope than I was originally led to believe,” Dave says, a decade after his grim diagnosis.
An expert in treating neuroendocrine tumors, Dr. Reidy-Lagunes knew that Dave would be a good candidate for an emerging type of exquisitely targeted therapy called theranostics.
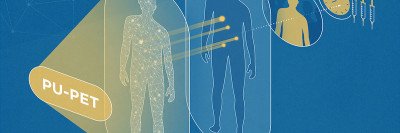
A mash-up of the words “therapy” and “diagnostics,” theranostics combines imaging techniques and targeted therapy into one very precise package. Like other forms of targeted therapy, theranostics involves a drug that circulates through the blood and binds to a specific target on cancer cells. But in this case, the drug is linked to a radioactive element that releases radioactivity to zap and kill the cancer cells. Unlike other targeted therapies, doctors can predict exactly where it will go.

Seeing Is Believing
“We have a theranostic motto, which is ‘We see what we treat, and we treat what we see,’ ” says nuclear medicine physician Lisa Bodei, who is Director of Targeted Radionuclide Therapy at MSK and who administered Dave’s treatments.
The emphasis on seeing is a key part of what distinguishes theranostics from other forms of precision medicine, and even chemotherapy.
Typically, when a patient receives a chemotherapy drug, for example, there’s no way of knowing how much of the medicine is getting to the cancer. With theranostics, it’s very different. There’s a visualization step at the beginning and at the end.
First, doctors administer a version of the drug carrying a diagnostic radioactive element, known as an isotope. This isotope emits a very low level of radiation that does not damage cells but can be easily visualized with a PET scan. This “tracer” allows the doctors to see where the drug is collecting.
Then, during the therapeutic phase, they switch to a version of the drug carrying a therapeutic isotope, which emits enough radiation to kill cancer cells. Doctors know exactly where it will go: the same place that the diagnostic tracer did. Moreover, they can do a follow-up scan to confirm. The end result is that doctors can see precisely where the drug went to kill cancer cells.

Dr. Bodei uses a military metaphor to describe the approach. “It’s like having a tank that is outfitted with both radar to survey the territory and a missile launcher. The tank operator detects the enemy and then attacks the enemy with the same tank,” she says.
The arsenal comes from around the world, according to MSK radiopharmacist Serge Lyashchenko, who oversees the manufacturing process of these powerful drugs. For each patient, he orders a particular radioactive isotope produced by a nuclear reactor. “Depending on the isotope needed, the reactor might be in Belgium, South Africa, Germany, or Turkey,” says Dr. Lyashchenko.
Under strict safety protocols in the basement of MSK’s Schwartz building near Memorial Hospital, he and his team create these drugs deploying radioactivity. “Basically, the radiation emitted by these drugs rips molecules apart,” Dr. Lyashchenko explains. “When it comes to cancer, the molecule you want to break is DNA, so that the cancer cells can’t reproduce, and they die.”
The process is so precise, the surrounding healthy tissue can be spared.
Though the idea of being exposed to radiation might sound scary, the directed nature of the treatment means that it is very safe. And compared with some other treatments that Dave Norkin has had, he says it’s been relatively painless.
“You just sit for three to four hours while they administer the medication,” he explains. “You really don’t feel anything.”
Dave received the first of several theranostics treatments while participating in a clinical trial, led by MSK physicians. It involved a radioactive version of a hormone called a somatostatin receptor (SSTR) antagonist. This hormone receptor is found in many neuroendocrine tumors and serves as the target for the theranostic agent.
Dr. Reidy-Lagunes notes that the trial showed that the treatment was safe and effective. “This therapy was a game changer for many of our patients,” she says. “Dave was our last patient enrolled, and it kept his tumor controlled for close to two years.”
That was especially striking to Dr. Reidy-Lagunes because no other therapies Dave had received had been able to achieve such good results.
“To have such a great response from an experimental theranostic drug was one of the most gratifying moments of my career,” she adds.
Since Dave participated in that clinical trial, he’s received other theranostics treatments that have kept his cancer in check.
What’s Old Is New Again
Theranostics has been in the news a lot recently, in part due to a recent FDA-approved theranostic imaging agent for prostate cancer and a treatment version that is currently being tested in clinical trials.

But Dr. Bodei points out that theranostics itself isn’t new. In fact, 2021 marks the 80th anniversary of the very first theranostic agent — radioactive iodine for the treatment of thyroid cancer. This is still the standard treatment for that disease.
Today, the cancers most commonly treated with theranostics are thyroid cancer, prostate cancer, and neuroendocrine cancers. But, in theory, it can be used against all kinds — provided scientists can identify good targets on cancer cells.
In recent years, researchers have gotten better and better at identifying unique targets on cancer cells and at devising small molecules or antibodies to deliver their toxic payload with greater precision and power.
The Next Wave
The most exciting development in theranostics is the use of a type of radioactivity called alpha emitters that are 100 times more powerful, according to Jason Lewis, Chief of MSK’s Radiochemistry and Imaging Sciences Service and the Emily Tow Jackson Chair.
“And when they deposit their lethal energy, it’s in the range of a few cells so we can spare healthy tissue,” says Dr. Lewis. “Alphas are really taking theranostics to the next level.”

Dr. Lewis is an expert in the development of radiopharmaceuticals for targeted diagnosis and the treatment of cancer. His research has been generously supported by the Tow Foundation and Marie-Noelle Meyer and her late husband, Fred J. Meyer.
In part due to Dr. Lewis’ expertise and persistence, MSK just got the green light to build a new facility so that these powerful agents can be made right on-site.
“We’re likely to be the first academic site in the US to have a laboratory dedicated to the clinical production of these agents,” he says. “That will help MSK both meet patient demand and also help place ourselves at the cutting edge of developments in this important area.”
Heiko Schöder, Chief of the Molecular Imaging and Therapy Service at MSK, emphasized what distinguishes MSK in the field: the focused collaboration of basic science researchers and clinicians to develop novel theranostics for more cancers.
Looking Ahead
Dave Norkin has far outlived his original doctor’s expectations. It will be 10 years in March since he was diagnosed.
“I know that when I get a certain treatment, it’s not going to last forever and that the cancer is going to recur,” Dave explains. “But hearing about all the new things they’re doing is really uplifting. I hope anyone reading this will be comforted knowing there are a lot of really good people trying their hardest to make sure we can live with this and enjoy our best lives.”
Advances in radiotheranostics are supported by The Tow Foundation, long-time contributors to MSK’s mission.