
Winners of Paul A. Marks Prize for Cancer Research Present Their Research at Scientific Symposium Held at Memorial Sloan Kettering Cancer Center
Young investigators were celebrated at the third biannual awarding of the Paul A. Marks Prize for Cancer Research. Introducing the 2005 winners to an enthusiastic, standing-room-only crowd, Memorial Sloan Kettering Cancer Center President Harold Varmus said, “The winners have some things in common… All three of them are interested in the molecular genetics of what we call cell signaling. They tend to work with cell cultures and mouse models. But as you’ll hear, in every case the investigators have paid special attention to the possible application of their findings to the treatment and diagnosis of human patients, or to the understanding of the pathophysiology of human cancer.” At a ceremony held in December in the Rockefeller Research Laboratories Auditorium, Dr. Varmus also acknowledged his pleasure that each winner had a strong connection to Memorial Sloan Kettering and its staff. Tyler E. Jacks received his PhD while working in Dr. Varmus’ laboratory (during Dr. Varmus’ tenure at the University of California, San Francisco) and is a member of the Center’s Board of Scientific Consultants. Scott W. Lowe has collaborated frequently with many members of the Memorial Sloan Kettering Cancer Center research community. Jeff Wrana completed his postdoctoral fellowship under the guidance of Joan Massagué, who was then Program Chairman for Cell Biology and is now Program Chairman for Cancer Biology and Genetics. The prize selection committee was chaired by Jeffrey M. Friedman, a professor at The Rockefeller University and a Howard Hughes Medical Institute (HHMI) investigator. The ten-member committee was made up of leading scientists from top research centers throughout the United States, including several previous Marks Prize winners.

Named for Dr. Marks, President Emeritus of Memorial Sloan Kettering Cancer Center, who led the Center for 19 years, from 1980 through 1999, the prize recognizes significant contributions to the basic understanding and treatment of cancer by scientists no more than 45 years old at the time they are nominated. The prize was created by Memorial Sloan Kettering Cancer Center’s Boards of Overseers and Managers at the time of Dr. Marks’ retirement to recognize his many contributions to cancer research, patient care, and education. The winners share a $150,000 award, and each receives a medal bearing Dr. Marks’ likeness.
Immediately before the symposium there was a luncheon honoring the winners, and a reception followed the three presentations at which attendees had the opportunity to talk to the winners about their latest projects.

Tyler Jacks
Dr. Jacks is the David H. Koch Professor of Biology at the Massachusetts Institute of Technology, Director of the MIT Center for Cancer Research, and an HHMI investigator. He has taken novel approaches to creating better, more accurate models of a variety of human cancers in mice. Much of his research has focused on the oncogene K-Ras, which is implicated in 30 percent of non-small cell lung cancers, 90 percent of pancreas cancers, and 50 percent of colon cancers, as well as many other tumors. He also has studied mutations of tumor suppressor genes. His laboratory has engineered mice in which researchers can precisely regulate where and when mutations of K-Ras, p53, and other cancer-causing genes are activated. This allows them to study cancer in its very earliest stages. “There are many applications for these mouse models,” Dr. Jacks said. “We can use them to identify biomarkers [such as proteins or peptides] that indicate the presence of disease, which will lead to the development of better screening tests for cancer. In addition we can use them to develop better imaging technologies, which can give us a very detailed understanding of how cancers first arise.” Developing these technologies is especially crucial for tumors such as those of the lung and pancreas, which in humans are usually diagnosed at a late stage when treatment is least effective.
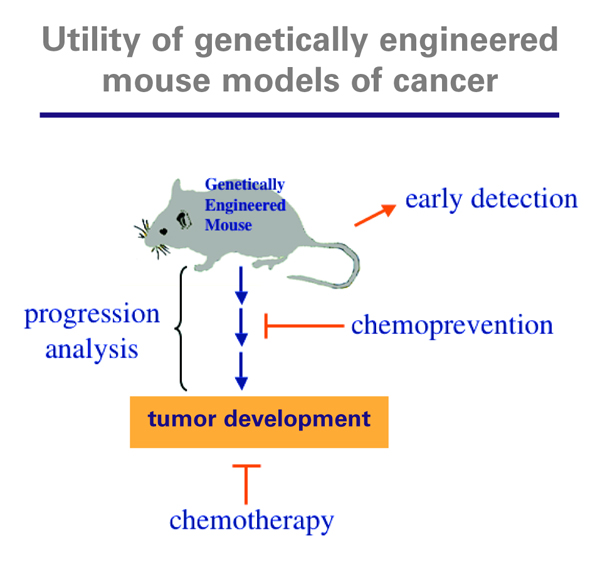
These mouse models can also be used to test various therapies, both conventional and experimental, including drugs that are targeted to block a particular pathway. “So far, our models have shown that certain drugs predicted to be effective in treating particular cancers are not, and these results have foreshadowed failures later seen in the clinic,” explained Dr. Jacks. Another of his goals is to develop better chemopreventive agents, which would eliminate cells before they become cancerous or before they spread.
Dr. Jacks received his PhD degree in biochemistry from the University of California, San Francisco, and completed a postdoctoral fellowship at the Whitehead Institute for Biomedical Research.
Scott Lowe
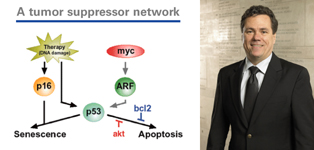
Dr. Lowe is a professor at Cold Spring Harbor Laboratory, Deputy Director of the Cold Spring Harbor Laboratory Cancer Center, and an HHMI investigator. He is exploring the molecular and genetic machinery of apoptosis (programmed cell death) and cellular senescence (in which cells irreversibly stop proliferating but remain alive). These normal cellular processes are disrupted in cancer cells, thus allowing them to grow and spread. Much of his work has focused on the tumor suppressor gene p53, which is mutated in about half of all cancers. His work has shown how changes in p53 can lead to the development of tumors and how the disruption of p53 can affect a tumor’s response to therapy, leading to drug resistance. Dr. Lowe’s work, like that of Dr. Jacks, has centered on the development of advanced, groundbreaking mouse models. “These models have allowed us to understand the evolution of cancer — how it progresses and responds to therapy,” said Dr. Lowe. One particular area of his research has been using these models to make sense of apoptosis and senescence so that these processes can be restored to cancer cells, thus allowing traditional chemotherapy drugs to destroy them. Another focus of his mouse model research is to gain insight into the genetic factors that influence the effectiveness of targeted therapies. “Our long-term goal is to use these models to determine how to make chemotherapy agents that are more effective and also to develop new combination therapies,” Dr. Lowe said. In his most recent research, Dr. Lowe has used the relatively new technique of RNA interference to more closely block the expression of genes in mice. Dr. Lowe received his PhD degree in biology from MIT and completed a postdoctoral fellowship there. Dr. Jacks was one of his mentors at MIT.
Jeff Wrana
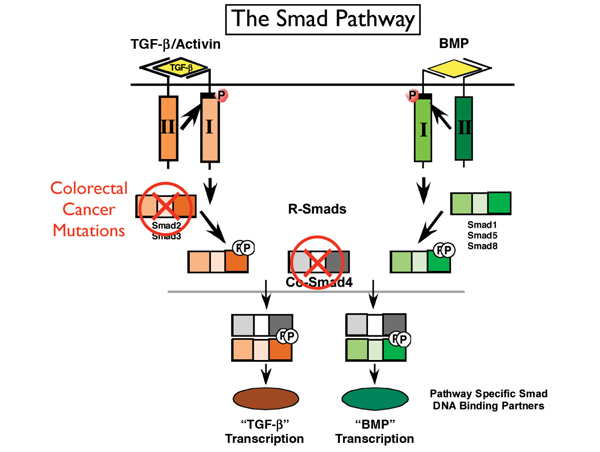
Dr. Wrana is a professor of medical genetics and microbiology at the University of Toronto and a Senior Scientist in the Program in Molecular Biology and Cancer in the Samuel Lunenfeld Research Institute, part of Mount Sinai Hospital. He is also an HHMI International Scholar. His work has focused on the transforming growth factor-beta (TGF-ß) family of cell signaling proteins that regulate cell growth and function. These proteins are secreted by cells, and then can act on those same cells that secreted them or on nearby cells and control their behavior. “TGF-ß is interesting from a cancer perspective because it can both block and promote cancer growth,” Dr. Wrana said. His laboratory helped to define the components of the TGF-ß signaling pathway and determine how its receptors are internalized by cells, a critical event that allows cells to function. His work demonstrating that a key component of the TGF-ß pathway is mutated in some forms of colorectal cancer was critical in establishing the role of this pathway in particular human tumors. Most recently, Dr. Wrana has begun using the technology of high-throughput screening (which allows the analysis of hundreds or thousands of proteins simultaneously) to study not only TGF-ß but other key signaling pathways as well. “These different pathways work together to create a code or language that tells cells how to behave,” said Dr. Wrana. “What is really important from a cancer perspective is not just the individual pathways but how the interaction of these pathways leads to cancer. This transformation becomes very complex as the cancer progresses and more mutations and more pathways become involved,” he said.

“My dream goal is to discover drugs that target the interactions of these pathways,” he added. The use of robotics and high-throughput technology will allow his lab to explore these interactions and test compounds in many different human cell lines.
Dr. Wrana received his PhD degree in biochemistry from the University of Toronto and completed a postdoctoral fellowship at Memorial Sloan Kettering Cancer Center.
