
At MSK, we are united by a relentless dedication to conquering cancer. We are one of 73 National Cancer Institute-designated Cancer Centers, with state-of-the-art science and technology supporting clinical studies, personalized treatment, and compassionate care for our patients. MSK has devoted more than 135 years to exceptional patient care, influential educational programs, and innovative research to discover more effective strategies to prevent, control, and ultimately, cure cancer.
Apply to our 2026 Cohort, focused on de-risking the path to the clinic, here.
The institution is home to an exceptional community of researchers and clinicians committed to advancing the cutting edge of cancer treatment, publishing pioneering findings from more than 130 laboratories and 1,800 active clinical trials. MSK’s combined scientific and clinical infrastructure is an incredible platform for innovation, to deliver more effective treatments to people with cancer.
The MSK Therapeutics Accelerator aims to establish close collaborations leveraging MSK’s expertise to de-risk critical points in the development of promising therapeutics. In particular, the Therapeutics Accelerator has a special interest in supporting treatments for rare diseases.
Among academic-research institutions, MSK is a leader in creating and developing innovation with impact. To date, global regulators have approved 15 therapeutics involving MSK inventions—and our institution has meaningfully contributed to the clinical development of many more.
For our industry partners, this accelerator program provides streamlined access to MSK facilities and infrastructure, as well as the opportunity to collaborate with our leading clinical and scientific experts through all stages of drug development.
The MSK Therapeutics Accelerator provides participants with a primary point of contact — managing the existing project while providing concierge support to streamline and accelerate the process. This facilitates additional engagement and work with other groups at MSK if/when such services are requested.
Other important benefits for Therapeutics Accelerator participants include committed support from MSK’s marketing team and IND writing and advising group. For Accelerator partnerships, MSK requests a pre-negotiated commercial stake in partner companies or co-developed products, which enables more flexible terms and faster contracting of individual projects moving forward.
Learn more about how the MSK Therapeutics Accelerator program helps pharmaceutical and biotech companies accelerate drug development.
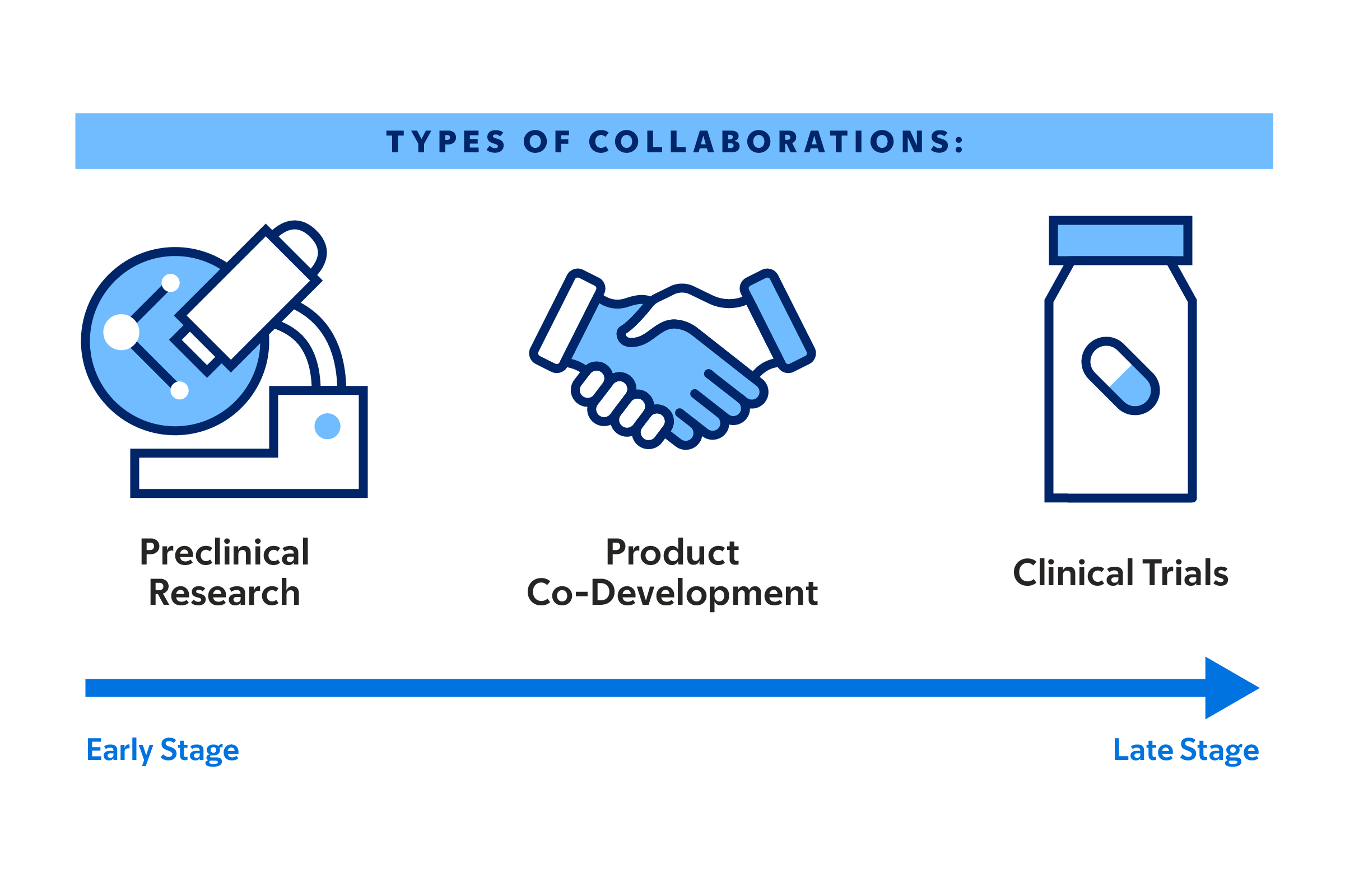
Through all stages of developing new cancer therapeutics, MSK aims to jump-start the innovation process, by providing select projects with access to our expert teams and extensive preclinical research, product co-development, and clinical trial resources.
We do not provide financial resources through this program; specific research budgets will be mutually agreed upon during the contracting process.
The MSK Therapeutics Accelerator connects MSK’s industry partners with valuable institutional resources including potential access to our 33 state-of-the art core facilities which provide services and technology to support research. Our cores provide the highest quality of scientific technology and research support services in rapid turnaround time, while operating in a cost-effective manner. Each of the MSK core facilities is operated by research experts in the technologies and services offered.
Depending upon the nature of the project, MSK Therapeutics Accelerator program participants may engage with resources such as:
- MSK Organic Synthesis Core Facility: This core facility performs medicinal chemistry and compound optimization to generate Phase 1-enabling small molecule compounds. Expertise available but not limited to developing complex carbohydrates, precursors to radioisotope labeled PET agents, cold labeling strategies, nanomaterials, antibody conjugation, and structure-activity relationship (SAR) analysis.
- MSK Antitumor Assessment Core: This core routinely performs PK/PD studies and Good Laboratory Practice (GLP) toxicology studies. It also evaluates therapeutics in in vivo disease models in support of IND applications. MSK’s centralized patient-derived xenograft (PDX) library offers access to a curated selection of PDX models developed from hospital samples; these are accompanied with clinical, histology, and genomic annotation to inform clinical development.
- MSK Gene Editing and Screening Core: This core offers extensive capabilities in RNA interference, CRISPR-Cas9 mediated gene editing, genetic perturbation screening services and cell line generation. It also supports early drug discovery with a high-content and high-throughput siRNA and chemical compound-screening facility, offering cell viability, biochemical, and cell imaging assays.
- MSK Biotherapeutics Core: This core routinely performs genetic engineering/transduction of patient cells, produces clinical grade vector, performs molecular analysis of gene transfer efficiency/transgene expression in patient cells, and conducts quality control of clinical reagents and patient cells. As one of the first cancer centers worldwide to treat patients with cell therapies, MSK has a proven ability to operationalize new cell therapies quickly and efficiently.
- MSK Clinical Grade Production Core: This core specializes in the manufacturing of therapeutics for early-stage clinical trials. It supports IND applications for small molecules, monoclonal antibodies, biologics, vitamins, and other therapeutics. It routinely performs monoclonal antibody purification, chelation of therapeutic antibodies for radiochemical labeling, and vialing/lyophilization of therapeutics. It also assists with enhancing the solubility of active pharmaceutical ingredients.
- MSK Product Development unit: This expert team is able to plan, guide, and support IND application filings and IND-enabling studies. The unit, which includes dedicated staff with significant expertise communicating with the FDA on novel therapeutics, can provide guidance on IND applications (ex: design of PK, safety, pharmacology, and toxicology studies) as well as CMC sections (ex: formulation development, process development/GMP manufacturing) for first-in-human trials.
- MSK Clinical Trials: MSK is a leader in cancer-related clinical trials, with an outstanding record in clinical trial operations and administration and extensive teams with expertise in oncology patient care, clinical research operations and compliance, and clinical trials nursing, finance, and contracting. The MSK Therapeutics Accelerator connects companies with clinical experts to develop clinical trial protocols for MSK’s specialized care setting and leverage the institution infrastructure for robust correlative studies.
- Access to Consented, De-Identified Clinical Data and Samples from extensive and invaluable sources that include longitudinal clinical outcomes and genomic data, including microbiome data, and the MSK Biobank.
The MSK Therapeutics Accelerator’s Cohort Program provides a structured runway for companies and MSK faculty to explore and define new collaborations.
The Cohort Program holds an annual application round during which interested companies can submit proposals for collaborating with MSK. Applications are evaluated with consideration for the potential impact of the therapeutic program, the company’s health and record, as well as the feasibility of co-development at MSK.
Selected companies are matched with MSK faculty according to their scientific and clinical needs. These companies benefit from one-on-one mentorship with a faculty sponsor and structured introductions to MSK’s translational and clinical facilities. Cohort programming also includes pitch development, media coaching, and in-person pitch showcases to broadcast companies to the broader MSK research community and key external stakeholders.
This collaborative program aims to define a concrete workplan for co-developing a participant’s therapeutic(s) at MSK, as well as the opportunity to transition into formal participation in the Therapeutics Accelerator.
2025 Cohort companies included:
- Antelope Surgical Solutions: website
- Australis Pharmaceuticals: website
- BriaCell: website
- DoriNano: website
- Eris Biotech: website
- Frezent Therapeutics: website
- Manhattan Biosolutions: website
- Rejuvenation Technologies: website
- Saccharo: website
- StimOxyGen: website
For more information on the MSK Therapeutics Accelerator program, including 2026 opportunities, or previous cohorts, reach out to Jonathan Dow, [email protected].
Wondering if the MSK Therapeutics Accelerator Program would be a good fit for your company’s therapeutics development focus? Reach out to Jonathan Dow, PhD, Senior Program Manager, to discuss potential areas for collaboration with MSK’s expert professionals.
In addition, the Therapeutics Accelerator’s Cohort Program works with promising companies to define possible collaborations through tailored faculty mentorship and introductions to the range of translational and clinical resources at MSK. If this structured curriculum is of interest, we welcome applications for the 2026 Cohort.
Standard Inquiry and Selection Process
- Start. Get in touch with MSK Therapeutics Accelerator by contacting Jonathan Dow, [email protected] to start the conversation. This program is open to applicants from startups as well as larger and more mature biotech and other healthcare companies.
- Evaluation & Matching. This is an opportunity for MSK and potential program participants to discuss the scope of a planned project and potential opportunities for collaboration and project advancement with MSK faculty drug development and disease area experts.
- Negotiations & Contracting. The nature and timing of these discussions will be project specific, but topics to be discussed will include research budget, IP and data rights, contractual obligations, and development/commercialization plans.
- Collaboration. While the terms of any collaboration will be highly project specific, it is anticipated that scoped activities will typically last between one-to-three years.
Since its 2024 launch, the MSK Therapeutics Accelerator has supported 10+ collaborations with promising preclinical- and clinical stage-companies. Read more about public collaborations at the following sources:
- MSK Expands Collaboration with Curadev Through MSK Therapeutics Accelerator Program | Memorial Sloan Kettering Cancer Center
- BriaCell Announces Collaboration with MSK Accelerator to Advance Bria-OTS+(TM) for Breast Cancer - BriaCell
- Saccharo Inc. Announces Collaboration with Memorial Sloan Kettering Cancer Center’s Therapeutics Accelerator
- Xintela announces subsidiary Targinta collaboration with MSK Therapeutics Accelerator
- Prodigy Biotech enters into a partnership with leading cancer center to advance products to improve outcomes for patients undergoing allogeneic HSC transplant
For questions about the MSK Therapeutics Accelerator or to apply, reach out to Jonathan Dow, PhD, Senior Program Manager, at [email protected].