The mammalian brain is an extremely complex structure both anatomically and functionally, that simultaneously integrates multiple inputs to produce an appropriate response (Sillitoe, 2007; Joyner & Bayin, 2022). Our studies are aimed at bringing together studies of neural development with analysis of the final circuitry and function of the adult brain. The neurons responsible for each circuit in the brain have a specific spatial organization that is optimized not only for the function of individual circuits, but also to allow long range circuits to interact with precision. How neurons become organized in 3D during development is a critical unanswered question. We have two main projects. In one we are identifying the signals that regulate scaling of the numbers of different cerebellar neurons during development to generate robust circuits, particularly those that communicate with the neocortex. In the second project we are exploring the degree to which the brain can be repaired after neuron loss and identify factors that can stimulate stem cells to regenerate neurons.
The cerebellum: complex morphogenesis yet simple circuitry
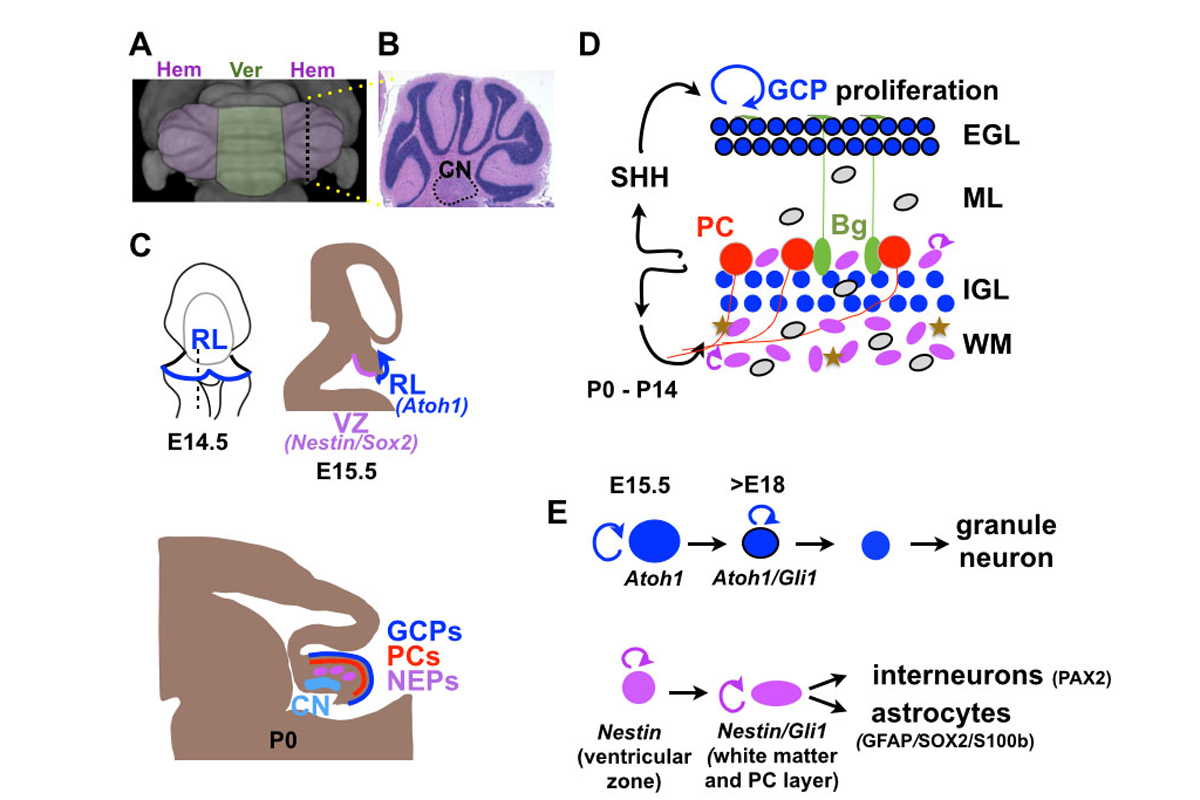
Figure 1: (A) Top view of MRI surface rendering of a mouse cerebellum with medial vermis (Ver) region and lateral hemispheres (Hem) color-coded. (B) H&E stained sagittal section of a hemisphere with cerebellar nuclei outlined (CN). (C) Schematic sagittal sections of developing brain showing ventricular zone (VZ), rhombic lip (RL), external granule cell layer (EGL) containing granule cell precursors (GCPs) and Purkinje cell (PC) layer. (D) Schematic showing GCPs (blue with black outline) in the external granule cell layer (EGL) and Nestin-expressing progenitors (NEPs; purple ovals) in the white matter (WM) and PC layer proliferating (round arrows) in response to SHH secreted by PCs. GCs migrate along fibers of Bergmann glia (Bg) to form the internal granule cell layer (IGL), NEPs produce interneurons (IN; grey ovals), astrocytes (brown stars) and Bg (green ovals). (E) Rhombic lip (Atoh1) and ventricular zone (Nestin) lineages indicating genes expressed in different cell types.
The cerebellum, consisting of 80% of the neurons in the human brain, is critical for skilled motor performance, and also participates in cognitive and social functions. As the cerebellum is the only brain region in rodents with a foliated structure (Fig. 1A), one aspect of our research is to identify cellular and genetic events that regulate the highly stereotyped process of foliation, or gyrification seen in the human neocortex (Sgaier, 2005; Sudarov, 2007; Legué, 2015, 2016; Lawton 2019). At the cellular level, the cerebellum consists of a dozen major cell types organized into a layered folded cortex with 3 pairs of cerebellar nuclei (CN) at its base (Fig. 1B). The developing cerebellum is particularly vulnerable to clinical and environmental factors, since much of growth in humans occurs in the third trimester and continues for a year after birth (Wang, 2014), and for three weeks after birth in mouse (Movie 1). Unlike other brain regions, the cerebellum has multiple progenitor zones (Leto, 2015; Joyner & Bayin, 2022)(Fig. 1C-E). The ventricular zone gives rise to all the GABAergic (inhibitory) cells including Purkinje cells, whereas the glutamatergic (excitatory) neurons, including the output neurons of the cerebellar nuclei and granule cells, are derived from the upper rhombic lip. Unique to the cerebellum, interneurons and astrocytes are generated from Nestin-expressing progenitors (NEPs) that leave the ventricular zone and continue to proliferate in the cortex after birth. The rhombic lip first generates neurons of the cerebellar nuclei at embryonic day (E) 10-13 in mouse, and then granule cell progenitors that migrate over the surface of the cerebellum to form a transient amplifying stem cell population of granule cell progenitors that undergo tremendous expansion of cell numbers for two weeks in mouse through symmetric cell divisions (Legué, 2015). The Purkinje cells express sonic hedgehog (SHH), a mitogen necessary for granule cell precursor expansion and also for expansion NEPs and production of interneurons and glia. Purkinje cells also integrate all the incoming information to the cerebellum, modulated by granule cells and interneurons, and relay the information to the cerebellar nuclei neurons that instruct the rest of the brain to modify behaviors. The Purkinje cells thus act as a critical hub that developmentally scales production of all the cells in the cerebellar cortex and functionally links all the cells of local and long-range circuits.
Principals underlying proportional scaling of neurons
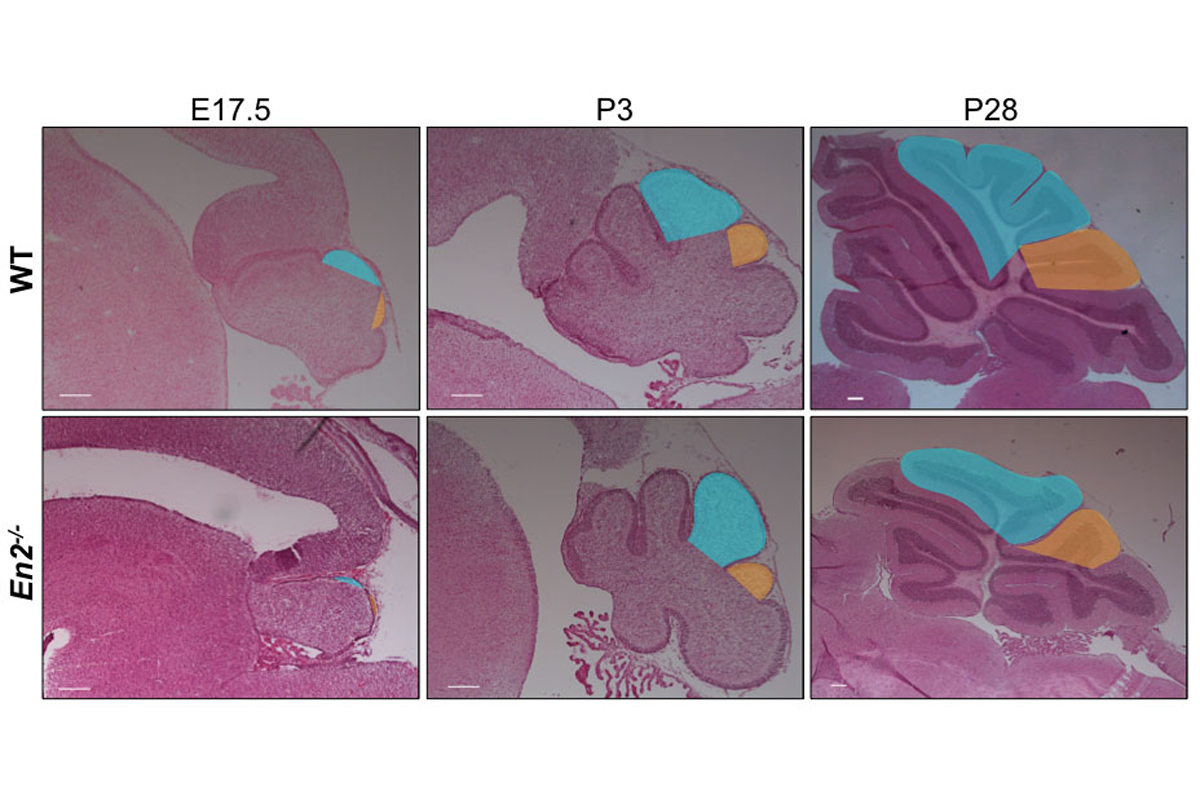
Figure 2. Sagittal sections of wild type and En2 null mutant cerebellum at the stages indicated illustrating how lobule 8 (shaded orange) in the mutant is reduced in size and there is a delay in foliation.
In one project, we have utilized the genes encoding the engrailed homeobox transcription factors (En1 and En2) as an entry point to study cerebellar growth, circuit formation and patterning, as conditional mutants have defects in all these processes (Joyner, 1991; Sillitoe, 2008, 2010; Joyner, 1991; Sgaier, 2007; Orvis, 2012; Cheng, 2010; Legué, 2016). Our current studies are based on our finding that while growth of particular folds are reduced in En1/2 mutants, the basic cytoarchitecture and scaling of different neurons is normal (Fig. 2). Consequently, there are regionally defined reductions in the number of cerebellar nuclei neurons and Purkinje cells before birth, and a subsequent reduction in SHH signaling and generation of granule cells (Willett, 2019). We are currently testing whether experimental ablation of particular subsets of cerebellar nuclei neurons results in loss of circuit-related Purkinje cells, whether neurotrophic factors expressed by cerebellar nuclei underlie the Purkinje cell phenotype, and defining the circuits linking the cerebellum and neocortex, and are generating mice expressing tagged-EN2 protein for molecular studies to identify key target genes. We also have developed a method for live imaging of fluorescently labeled cells in slices of the developing cerebellum. In one set of experiments, we are using the approach to study aspects of proliferation and migration of granule cell precursors that underlie foliation (Movie 2). Our long-term goal is to understand how cerebellar circuits regulate neocortex initiated behaviors. Since sequence changes in human EN2 have been linked to autism (Benayed, 2005), and En2 mutant mice have deficits in social behaviors (Brielmaier, 2012; Crawley, 2012), a better understanding of how the En1/2 genes regulate cerebellum development and modulate behaviors should provide insight into human cerebellar malformation syndromes with gross morphological changes, as well as diseases such as autism that could have significant cerebrocerebellar circuit dysfunction.
Adaptive reprogramming of Nestin-expressing stem cells during regeneration
In a second project, we have uncovered that the mouse neonatal cerebellum has a large capacity to regenerate neurons killed around birth. In one study we demonstrated that Purkinje cells killed a day after birth can be replaced by proliferation of a rare population of immature Purkinje cells (Bayin, 2018). In a second series of studies we showed that ventricular zone-derived cells expressing the stem cell marker Nestin in the cerebellar cortex have the ability to change their normal fate (glia) and become rhombic lip-derived granule cells in response to injury (Wojcinski, 2017; Wojcinski, 2019; Yang & Joyner, 2019). Using genetic ablation (Cre/loxP) or irradiation of the early postnatal mouse brain to eliminate most of the granule cell precursors, we found a remarkable regeneration of the granule cells. Moreover, using genetic labeling and fate mapping (with FlpoER; see Fig. 4) we identified Nestin-expressing progenitors (NEPs) in the cortex that rapidly respond to the injury by expanding, migrating to the granule cell progenitor niche and adopting a granule cell molecular profile. We developed a method for live imaging of fluorescently labeled cells in slices of the developing cerebellum and used the approach to study aspects of proliferation and migration of granule cell precursors (Movie 2) and to image fluorescently labeled-NEPs to prove that NEPs migrate to the granule cell progenitor layer (Movie 3). A second population of NEPs responds to the injury by delaying production of interneurons and astrocytes, thus maintaining circuit cell proportions, and rescuing motor behaviors. Most recently, single cell sequencing (scRNA-seq) was used to identify genes that underlie adaptive reprogramming of NEPs. We uncovered that the neurogenic transcription factor ASCl1 must be upregulated by gliogenic NEPs for them to change their fate to neurons (Bayin, 2021). Additional projects include determining whether NEPs in the adult cerebellum can be stimulated to replenish cells lost due to injury and testing the requirement of ROS signaling for driving adaptive reprogramming.
Videos
- MRI surface rendering of developing mouse brain viewed from the back showing how the cerebellum folds and grows from 1 to 11 days after birth (adapted from Szulc, 2015).
- Live imaging of a cerebellar slice from an E18.5 Atoh1-CreER; R26LSL-tdTom mouse given tamoxifen two days earlier showing movement of fluorescently labeled granule cell precursors (green) in the external granule cell layer (EGL). The white line marks the outer edge of the cerebellum. Images taken every 3 minutes for 1.5 hours. See Figure 4 for explanation of fate mapping using R26LSL-tdTom mice and tamoxifen.
- Live imaging of a cerebellar slice from a P6 Nestin-Cfp/+ transgenic mouse. Cyan florescent protein (CFP; white) labels the cytoplasm of NEPs. A NEP in the Purkinje cell layer is marked with a blue dot and migrates to the external granule cell layer (EGL) and undergoes cell division during adaptive reprogramming following ablation of granule cell precursors in the EGL. Normal NEPs stay in the Purkinje cell layer (Wojcinski, 2017). The green line indicates outer edge of the cerebellum (under the EGL). The magenta line indicates outer edge of the Purkinje cell layer where the NEPs reside. Images taken every 3 minutes for 4.5 hours.