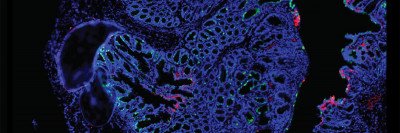
At a worldwide gathering of the American Association for Cancer Research (AACR), Memorial Sloan Kettering Cancer Center (MSK) scientists presented new insights into the connection between cancer and the gut microbiome, often referred to as the “forgotten organ.” Their findings included:
- Clues that microbiomes might explain the increase in colorectal cancer among younger patients.
- Discoveries about how patients’ recovery from bone marrow transplants can be affected by their microbiome and the amount of fiber in their diet.
Studying the Connection Between the Gut Microbiome and Gastrointestinal Cancers, Especially in Younger People
In one study, researchers found a difference in the gut microbiomes of people diagnosed with colorectal cancer before age 50 and those after age 50.
This work was led by physician-scientist and gastrointestinal oncologist Karuna Ganesh, MD, PhD, and gynecological oncologist Melissa Lumish, MD. Dr. Ganesh, a medical oncologist specializing in the treatment of gastrointestinal cancers, also studies them in her lab. Her work investigates why these cancers metastasize (spread) to other parts of the body.
Dr. Ganesh says: “In recent years, we have been seeing younger and younger patients develop GI cancers, for reasons that are not explained by tumor genetics. We hypothesize that changes in environment and lifestyle factors, reflected in the bacteria that live inside us, may provide clues to what’s going on.”
Explaining Why GI Cancers Are Increasing in Younger People
The MSK team reported data on 53 patients with colorectal cancer and 23 patients with esophageal cancer.
For both groups, they collected “living” samples of tumor as well as normal tissue, blood, and stool bacteria from people who had been newly diagnosed with stage 3 disease and those with stage 4 disease. In the first part of the study, the team looked at whether differences in the microbiome samples might explain why certain people are more likely to have their disease progress. They didn’t focus on individual strains of microbes, but instead looked at the variety of bacteria that were present. In this part of the study, they didn’t see a marked difference.

However, when they divided the patients by age rather than stage of disease, they did find noticeable differences. The populations of bacteria in people who had early-onset cancers (developing before the age of 50) were not the same as they were in those who developed cancer at a later age. “These differences in bacteria might explain why these patients develop cancer at a younger age,” Dr. Lumish says.
Dr. Lumish adds that this research is still in its early stages and much more data collection is needed. For example, researchers would need to compare microbiome samples in those with early-stage GI cancers to those of people the same age who don’t have cancer. “Additionally, we know there are microbiome differences based on where people live,” Dr. Lumish says. “But we find this trend toward increased early-onset cancers across the globe.”
Understanding Why GI Cancers Progress
These findings suggest new areas of research for Dr. Ganesh to pursue in her lab, which is part of the Molecular Pharmacology Program at the Sloan Kettering Institute. Her lab has developed novel multi-tissue three-dimensional “organoid” models that re-create the interactions among cancer cells, immune cells, and microbes in a dish. “These models help us study how these three components are talking to each other and how this enables cancers to grow and become more aggressive,” Dr. Ganesh explains.
The team is reconstituting the tumor cells, gut bacteria, and immune cells from individual patients as multi-tissue organoids and studying how they interact. The investigators will also follow the patients in the study and collect additional samples. They are tracking whether changes in the microbiome might explain why the type of cancer a patient has changes — for example, if it no longer responds to treatment or advances from stage 3 to stage 4.
“About half of people with stage 3 colorectal cancer are cured of their disease, while the other half relapse and eventually die from it,” Dr. Ganesh says. “We hope that, through our lab research, we can improve outcomes for all patients.”
Studying How Dietary Fiber and Microbiota Affect BMT Outcomes
Another study looked at whether dietary fiber can improve outcomes for people receiving a bone marrow transplant (BMT) when using donor cells (allogeneic). The team was led Jenny Paredes Sanchez, PhD, a research scholar in the lab of by Marcel van den Brink, MD, PhD, in collaboration with BMT expert Jonathan Peled, MD, PhD, who leads a laboratory at MSK devoted to studying the role of the intestinal microbiome in people with cancer.

The scientists analyzed data from 173 people having BMTs at MSK. The researchers collected details on everything the patients ate as well as regular stool samples to analyze their microbiomes. They found that patients who consumed more fiber had a greater diversity of bacterial strains in their guts — a sign of good gut health. One of the goals of the study is to ask whether there is a relationship between fiber intake and graft-versus-host disease (GVHD). GVHD is a potentially fatal complication of BMTs in which the donor’s immune cells attack the patient’s healthy tissues.
“When we think about the impact of diet on the microbiome, we can consider it a kind of drug,” says Dr. van den Brink. “If we want to learn how to maximize the potential benefits from a certain diet, we need to do a better job of monitoring what people eat when they are recovering from BMTs.”
“Nutritional studies have been integrated into cancer research in the past, but this project used a much more rigorous approach,” Dr. Paredes says.
The Link Between Fiber and Microbiome Diversity
The investigators took what they observed in the clinic into the lab to learn more, using animal models of allogeneic BMTs. In the lab mice, the investigators focused on cellulose, a type of dietary fiber that’s found in raw vegetables, grains, and legumes like lentils and chickpeas.
“There have been a lot of studies on the benefits of fiber in general, but we decided to focus on one particular type,” Dr. Paredes explains. “Cellulose has a big impact on the microbiome, because humans don’t have the enzymatic machinery to break it down. We completely rely on our microbes to digest it.”
The researchers found that mice that consumed larger amounts of cellulose had more diversity in their microbiomes. This included beneficial strains that are known to produce a type of molecule called short-chain fatty acids. Previous studies from Dr. van den Brink’s lab have shown that these molecules protect the lining of the gut, helping to prevent GVHD. The presence of beneficial strains can also help prevent more harmful strains that can cause serious infections from taking over.
Both studies presented at AACR benefited from the Molecular Microbiology Facility, which was founded at MSK in 2010 to collect, process, and analyze human and mouse samples to analyze the microbiome.
Translating Lab Findings to Clinical Trials for BMT Patients
Dr. van den Brink notes these findings are important, but only the beginning of understanding how to help patients having BMTs. “Just like with a drug, we need to do clinical trials to determine the best ‘dose’ of fiber,” he says. “In the mice, we found that too much cellulose in the diet also caused problems.”
He adds that some studies have also suggested that short-chain fatty acids have a positive effect on the immune system, including promoting the growth of immune cells that protect against inflammation. “We are thinking about developing a trial to look at a lot of these things,” he says. “What we are learning in the lab can help to guide these studies.”