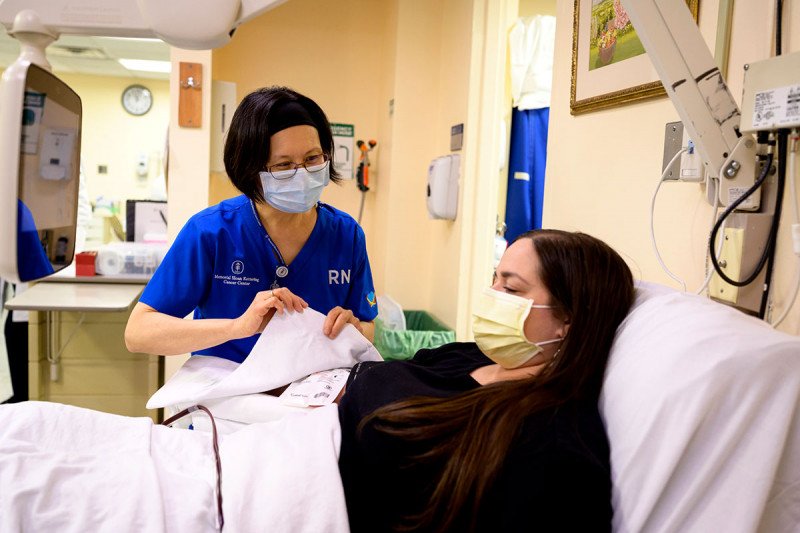
Lisa Toth donates plasma with help from Anli Chang.
As the world struggles with the epic challenge of the COVID-19 pandemic, employees at Memorial Sloan Kettering have risen to the occasion in countless ways. In addition to providing extraordinary care and comfort for patients with COVID-19, our staff members are contributing to our collective understanding of a new treatment approach using convalescent plasma — by both studying it and donating it.

Esperanza Papadopoulos
Plasma from people who have recovered from a viral infection has long been known to have potentially therapeutic benefits for patients. Now, hundreds of MSK employees who have recovered from COVID-19 have stepped forward to donate their plasma to treat patients through a clinical trial led by hematologic oncologist Esperanza Papadopoulos, Clinical Director of the Adult Bone Marrow Transplantation Inpatient Unit, who is the study’s principal investigator. Genovefa Papanicolaou, an infectious diseases specialist; Cheryl Goss, an associate attending in Laboratory Medicine, and Cy Wilkins, a benign hematologist, round out the leadership team for the initiative. The group is working closely with staff from such specialized areas as clinical research, infectious diseases, nursing, laboratory medicine, hematology-oncology, health informatics, and more.
A National Emergency
On March 21, Sergio Giralt, Deputy Division Head of the Division of Hematologic Malignancies, and Paul Hamlin, Medical Director of the David H. Koch Center for Cancer Care at Memorial Sloan Kettering Cancer Center, took part in the first conference call of the National COVID-19 Convalescent Plasma Project. The virtual meeting included more than 100 participants from academic institutions who had come together to investigate the use of plasma as a treatment for people with COVID-19. After the meeting, Dr. Hamlin reached out to Drs. Papadopoulos, Papanicolaou, Goss, and Wilkins and asked them to lead MSK in this national grassroots effort, which now involves 57 institutions from 46 states.
“We immediately started to pull a team together,” says Dr. Papadopoulos. The task was mammoth. As quickly as possible, they had to launch a clinical investigation that would explore questions surrounding access, safety, and healthcare uses for the plasma donations. And they had to do it during a national health emergency while MSK was grappling with an altered workplace, with a greater proportion of employees working remotely or sick themselves.
The US Food and Drug Administration took steps to allow institutions across the nation to enroll multiple patients. This action significantly sped up the delivery of convalescent plasma to people with COVID-19. It also allowed researchers to launch clinical trials at a faster rate than usual.
“At this phase, we are investigating if we can get the plasma to patients who need it and whether there are any adverse effects,” says Dr. Papadopoulos. “The true efficacy of convalescent plasma will be explored in future trials.”
One early logistical question was where to get convalescent plasma. “Most hospitals in New York City have to rely on the New York Blood Center or the Red Cross for blood products, including plasma, because they don’t have donation facilities,” says Dr. Goss. “We’re fortunate at MSK to be one of only two healthcare institutions in the city with our own donor room.”
MSK’s donor room is located on the ground floor of the Arnold and Marie Schwartz Cancer Research Building. A full range of services for the collection, processing, and testing of standard blood components is available there. Given that capability, as well as the more than 1,000 employees who had tested positive for COVID-19 by early April, it made sense to keep collection efforts in-house and rely on plasma from MSK’s recovered employees, obtained in our own donor room.
Putting Plans into Action
A frenzy of preparation soon began.
“We don’t typically collect large amounts of plasma,” explains Dr. Goss. “We mostly collect platelets and red blood cells for our patients, so large-scale plasma collection required a huge ramping up of the donor room. It wouldn’t have been possible without the incredible efforts of the staff.”
Joann Tonon, who manages MSK’s blood, created entirely new workflows to handle the specialized process. Nurse leader Eileen Walsh then wrote and implemented multiple procedures for the new workflows. Collection instruments were reprogrammed for plasma collection. Large-volume collection kits were purchased as well.
At the same time, work was underway to identify employees who were willing to donate. The research team spread the news about the need for donors using various internal channels and word of mouth.
“Once employees heard about it, we had an overwhelming response,” says Dr. Papanicolaou.
As donors started to volunteer, physician assistants Theresa Elko and Lauren Levy screened hundreds of people and made thousands of phone calls. Joseph Licata, manager of the Blood Donor Program, worked tirelessly to schedule donors.
By April 13, when the first plasma donation was made, more than 500 MSK employees who had recovered from COVID-19 came forward to donate. Donors must meet a high bar for donation: They must have recovered from COVID-19, test negative for the virus, and have been without symptoms for at least two weeks. They must also meet the standard eligibility criteria for blood donation.
Despite the challenges, the team’s quest for plasma has been successful. Since April 13, they have collected 60 donations. Most important, 49 MSK patients with COVID-19 have received convalescent plasma.
One of those who donated is Lisa Toth, a nurse in one of the chemotherapy suites at the Rockefeller Outpatient Pavilion. Ms. Toth had the virus, recovered at home, and returned to work in mid-April.
“When I heard about the plasma donation opportunity, it wasn’t even a question if I would donate,” she says. “I really wanted to do anything I could to help my colleagues and the patients fight this virus. I’m grateful I was given the opportunity to donate.”
An Appreciative Recipient
One of the first patients to receive the new therapy was Yu Chen, an MSK medical oncologist who specializes in the treatment of prostate cancer and bladder cancer.
“I feel so grateful for the remarkable care and support I received from the MSK community,” says Dr. Chen, who is continuing his recovery at home after being released from Memorial Hospital on April 23.
Dr. Chen remembers “images of my colleagues hovering over me” while he was in the ICU, his status declining by the day. Tobias Hohl, Chief of the Infectious Diseases Service — and Dr. Chen’s former classmate — suggested he try convalescent plasma therapy. As his condition worsened, and while he was “praying not to be intubated,” he says, Dr. Chen was infused with plasma from a donation received the day before. He experienced rapid improvement in his symptoms, left the ICU three days later, and was discharged from the hospital five days after that, thankful for what he calls the “selfless and lasting gift” he had received.
Reflecting on his experience, Dr. Chen says, “I want to thank my donor from the bottom of my heart. I know when I’m cleared for work, donating myself will be my first task.”
The Prize: Antibody-Rich Plasma
Researchers don’t yet understand why convalescent plasma seems to help some patients and not others. They believe it may be connected to the level of antibodies in the plasma, which can vary greatly. Another puzzle is why one donor’s plasma may have a high titer, or concentration of antibodies, whereas another’s may be so low that it barely registers. In the fight against COVID-19, plasma with a high titer is the weapon everybody wants.
Knowing whether donated plasma has any antibodies only became possible at MSK the week of April 27. The Department of Laboratory Medicine evaluated three antibody tests from outside vendors. They determined that one submitted by Abbott Diagnostics was the best for use at MSK.
“It was an extensive evaluation process,” says Melissa Pessin, Chair of the Department of Laboratory Medicine. Dr. Pessin asked Ellinor Peerschke, Vice Chair of the Department of Laboratory Medicine, and Lakshmi Ramanathan, Chief of the Clinical Chemistry Service, to lead the effort. “We took samples of pre-COVID-19 blood that were collected last year and put them through the tests to ensure that the results didn’t cross with other coronavirus strains,” says Dr. Pessin. They also reached out to Deborah Korenstein, Chief of the General Internal Medicine Service, for help getting samples of serum from recovered employees, “to confirm that the tests worked as they were supposed to,” Dr. Pessin says.
Dr. Pessin says that the group is now working to see if the test can be modified to provide the actual titers of antibodies.
Going Forward
There’s no end date for the convalescent plasma trial. As antibody testing improves, researchers may become able to determine the level of antibodies a patient is receiving. Then additional trials may launch to explore whether convalescent plasma is an effective treatment.
Meanwhile, the team continues to collect plasma from the MSK staff, who are thankful for the chance they’ve been given to help COVID-19 patients.
“I have been staggered by the strength of my colleagues and patients — who are sometimes one and the same — and I am so grateful for this opportunity to explore new therapies for our patients,” says Dr. Papadopoulos.
As the principal investigator on the trial, she participates in regular meetings of the COVID-19 Convalescent Plasma Project, often involving 200 to 300 people. She also fields requests from MSK colleagues wondering if their patients might be eligible for the trial. She and the rest of the team are mindful of the unique roles they are playing in this unprecedented time — and the opportunities it has presented.
“I’ve been at MSK for more than 30 years, and I’ve never been exposed to such a broad cross-section of the institution or met as many people as I have since this trial started. It’s been exhilarating and humbling all at once,” says Dr. Papadopoulos.