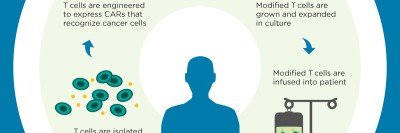
The Cellular Therapeutics Center at MSK has specific goals, including:
- to responsibly and safely conduct clinical trials of tumor-targeted T cells for the treatment of both hematological and solid tumor malignancies.
- to assess and optimize safety of chimeric antigen receptor (CAR) T cell therapies as well as understand the mechanisms associated with observed toxicities.
- to design and conduct first-in-human clinical trials utilizing genetically modified T cells based on novel discoveries made in the laboratory at MSK including the development of novel CAR vectors and combination therapies with small molecules or monoclonal antibodies.
- to study clinical data from clinical trials to assess biomarkers of response and mechanisms of resistance to CAR T cells.
- to develop a multidisciplinary program focused on the clinical development of cell-based therapies for cancer with an emphasis on CAR T cells.
- to train personnel to help develop multidisciplinary CAR T cell programs at academic medical centers and participate in multicenter CAR T cell trials.
- to advance the field of adoptive cellular therapy.