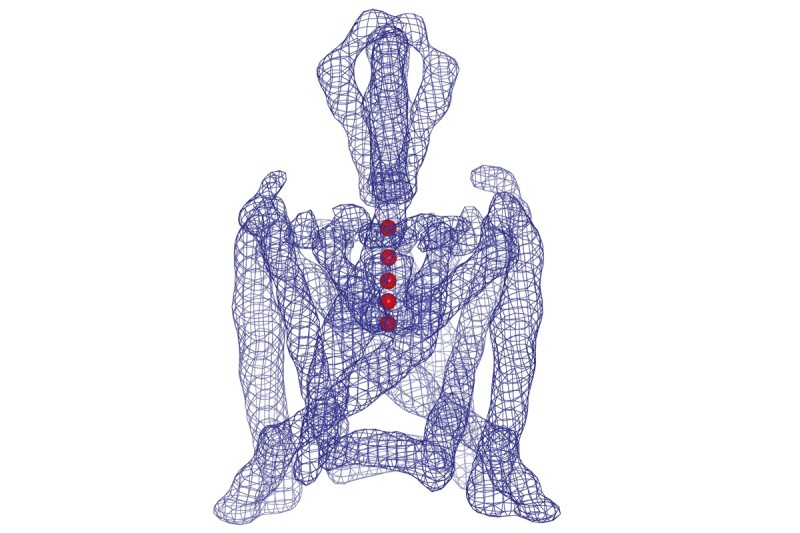
The protein K2P1 (blue) regulates the flow of potassium ions (red) in and out of cells. Memorial Sloan Kettering investigators revealed its three-dimensional structure using x-ray crystallography.
For the first time, Memorial Sloan Kettering investigators have revealed the three-dimensional shape of a protein called K2P1, which regulates the flow of potassium ions in and out of cells.
K2P1 belongs to a group of proteins called potassium channels, which help maintain a variety of critical body functions, including the transmission of nerve signals. Published in the journal Science on January 26, the study could shed light on how potassium channels function and guide the development of some types of drugs, including painkillers.
In an interview, Stephen B. Long, who heads a laboratory in the Structural Biology Program of the Sloan Kettering Institute, talked about the study, which he performed together with Alexandria Miller, a student of the Gerstner Sloan Kettering Graduate School of Biomedical Sciences.
What are potassium channels, and why do they interest you?
All the cells in our body are enclosed by a membrane, which separates a cell’s contents from its surroundings. Proteins called potassium channels form tiny pores in this membrane. Through the pores, potassium ions can enter and exit the cell.
The activity of potassium channels propels some of the body’s most vital functions, making these proteins an intense and fascinating area of research.
By regulating the flow of electrically charged potassium ions out of a cell, potassium channels establish a negative voltage inside the cell that is critical to the way our bodies function. For example, this voltage generates electrical signals in the nervous system, allowing individual nerve cells to communicate with one another.
What type of insight does your study provide?
My colleagues and I have drawn the first detailed, three-dimensional map of the potassium channel K2P1. By studying this map atom by atom, we and other scientists will be able to come up with specific ideas about how the channel works, and then test such ideas in the laboratory. Our report also gives clues about how the ion channel could be manipulated pharmacologically.
Will the findings ultimately benefit people?
It’s too early to tell with certainty. However, I think there are good reasons to be hopeful that our study will guide the development of certain types of drugs in the future.
For example, it is known that some currently used drugs, including antidepressants and painkillers, act by binding to K2P1 or to other potassium channels that resemble K2P1. Understanding the structure and function of the protein might be a first step toward improving the effectiveness of such therapies or limiting their potential side effects.
How was the research done?
The K2P1 protein is 30,000 times smaller than the width of a human hair, making it invisible even under the most powerful microscope. To visualize it, my colleagues and I used a technique called x-ray crystallography.
First, we produced large amounts of the protein, purified it, and concentrated it to the point that it formed crystals — solid structures in which all the atoms of K2P1 are symmetrically lined up.
Next, we shot high-energy x-rays at the crystal using a huge machine called a synchrotron. As such x-rays interact with and exit a crystal, they bounce off, or diffract, in different angles. Captured on a screen, the diffracted rays form a unique pattern, from which we were able to derive information about the clouds of electrons that make up the protein, rendering its unique shape.
We then used computer software to build an atomic model — a map that shows every atom of K2P1 in 3D.
What motivates you as a scientist?
The type of work we do in my lab requires a lot of time and perseverance. For example, it took us more than two years to obtain a K2P1 crystal we could work with. Sometimes, however, we get to be like the first men on the moon — in our own, modest way.
We were the first people to see what K2P1 looks like. The sheer beauty of the channel, and the notion that our bodies are continuously using it — even now, as we speak — really fills me with a sense of wonder. And now, we get to share our observations and our excitement with others, which is just as gratifying.

