Chimeric antigen receptor (CAR) T cell therapy has shown great promise for the treatment of certain blood cancers, like leukemia and lymphoma. By contrast, using this method to treat solid tumors has been more challenging.
On March 31, 2019, Memorial Sloan Kettering researchers presented results from a phase I clinical trial of CAR therapy in mesothelioma. This hard-to-treat cancer usually affects the tissue surrounding the lungs, called the pleura.
According to Prasad Adusumilli, a physician-scientist at MSK who is leading the trial, the CAR therapy proved safe and showed signs of efficacy in a small number of patients. Dr. Adusumilli presented these results at the annual meeting of the American Association for Cancer Research, held this year in Atlanta. The study was featured in the meeting’s press program.
“Traditionally, these patients with advanced-stage solid tumors have had poor outcomes despite aggressive treatment,” Dr. Adusumilli says. “These results are encouraging for patients who have limited treatment options.”
Study Details

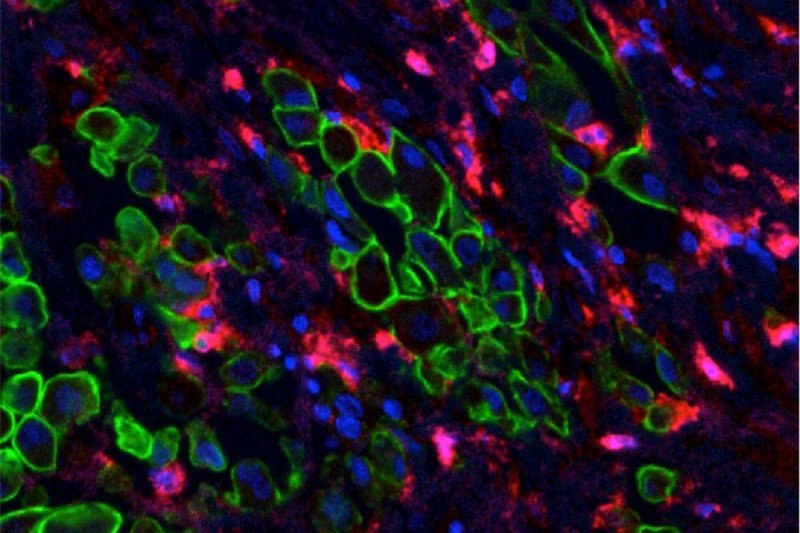
This trial included 21 patients: 19 with pleural mesothelioma, one with metastatic lung cancer, and one with metastatic breast cancer. They received CAR T cells built to target a molecule called mesothelin found on the surface of the cancer cells. The CAR T cells were infused directly into the space between the pleura.
A few weeks after the CAR T infusion, 14 patients received another immunotherapy treatment called pembrolizumab (Keytruda®). This drug, a type of checkpoint inhibitor, blocks a molecule on immune cells called PD-1 and boosts immune responses. Dr. Adusumilli and colleagues had previously shown that this combination was effective in treating cancer in mice.
Two patients who received PD-1-blocking drug had a complete response, as measured on PET scans, at 38 and 60 weeks after treatment. Five patients had a partial response, and four had stable disease.
Patients tolerated the CAR T treatment well. There were no CAR T–related side effects. Severe cytokine release syndrome, which is characterized by dangerously high fevers and has complicated CAR therapy for other cancers, did not occur in any of the patients.
“The combination of CAR T cell plus checkpoint blockade has been long awaited,” says Michel Sadelain, one of the pioneers of CAR T therapy. “This is the first study to support its possible efficacy in solid tumors.” The study represents 10 years of effort at MSK to develop a CAR therapy for solid tumors, he adds.
Expanding the CAR Fleet
MSK scientists developed the first effective CAR T cells for cancer. These prototype cells are built with an activity-boosting molecule called CD28 and target a molecule on B-cell leukemia and lymphoma called CD19. This cell-surface molecule is a good target for CARs because it is found on all B-cell cancers but not on other tissues that are critical for life.
Mesothelin is a potentially good target for CARs because it is present abundantly on solid tumor cancer cells but minimally on normal tissues. In addition to mesothelioma, mesothelin is found on pancreatic cancer, triple-negative breast cancer, lung cancer, and others. Drs. Adusumilli and Sadelain designed the mesothelin-specific CARs used in this study. The CAR T cells used in this study were engineered in MSK’s cell manufacturing facility, led by Isabelle Rivière.
“We are encouraged by these results,” Dr. Adusumilli says. “We hope they spur interest in the potential of CAR T cell therapy for solid tumors.”
Editor’s note: An earlier version of this article was published on May 17, 2018. It was updated with new information on March 31, 2019.