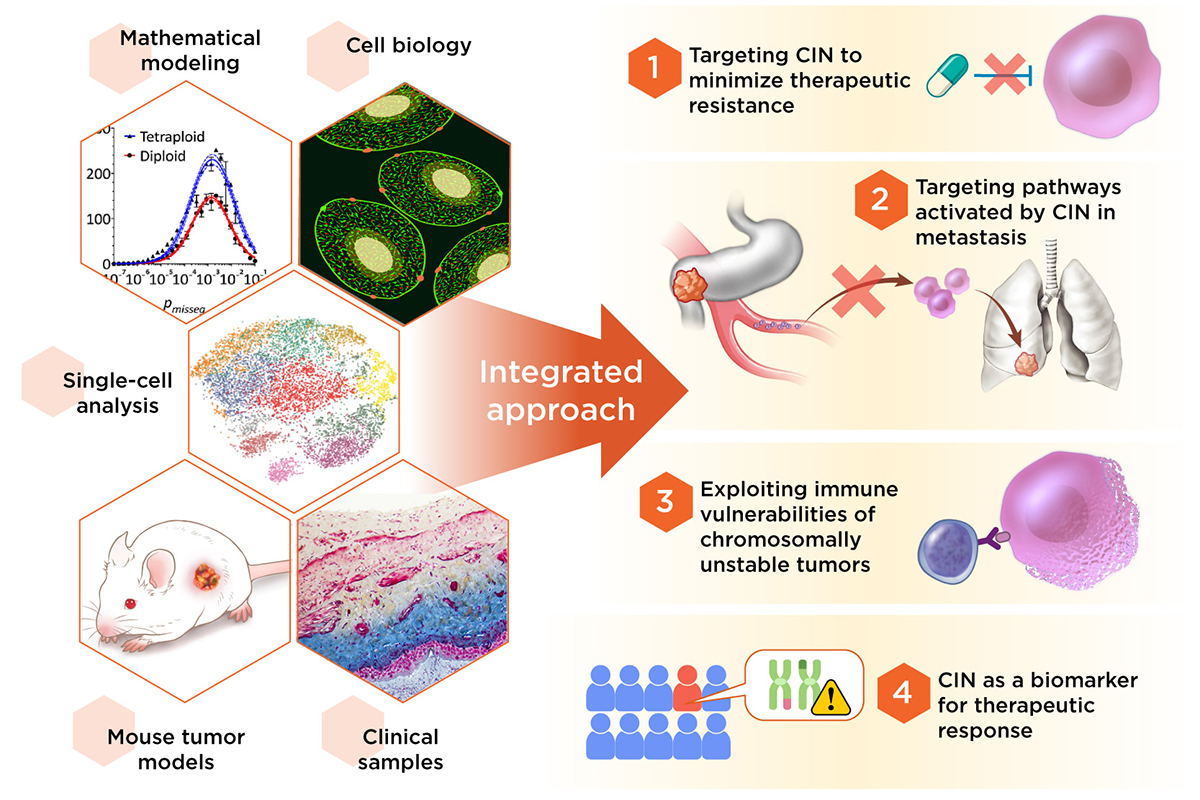
Our laboratory employs an interdisciplinary approach combining cell biology, biochemistry, as well as mouse and computational models to dissect the contributions of chromosomal instability (CIN) toward three key processes in human cancer: therapeutic resistance, immune evasion, and metastasis.
The role of CIN in cancer metastasis

We then found that the impact of CIN and chronic STING signaling is high dependent on the immune system. CIN-induced chronic activation of the cGAS–STING pathway promotes downstream signal re-wiring in cancer cells, leading to a pro-metastatic tumor microenvironment. This re-wiring is manifested by type I interferon tachyphylaxis selectively downstream of STING and a corresponding increase in cancer cell-derived endoplasmic reticulum (ER) stress response. Remarkably, treatment with STING inhibitors reduces CIN-driven metastasis in melanoma, breast and colorectal cancers in a manner dependent on tumor cell-intrinsic STING. Finally, we show that CIN and pervasive cGAS activation in micronuclei are associated with ER stress signaling, immune suppression and metastasis in human triple-negative breast cancer, highlighting a viable strategy to identify and therapeutically intervene in tumors spurred by CIN-induced inflammation (Li et al. Nature 2023). This work brings to light novel therapeutic strategies in otherwise aggressive metastasis-prone cancers. We are currently exploring the therapeutic utility of targeting CIN-activated pathways in the treatment of cancer metastasis and means of restoring pro-inflammatory signaling in tumors with CIN..
CIN and the tumor microenvironment
The ability of CIN to promote chronic inflammatory signaling highlights the intimate crosstalk between the cancer genome and the tumor microenvironment. How chromosomally unstable tumor cells not only survive, but thrive and metastasize, amidst chronic inflammation remains a mystery. We identified an extracellular metabolic pathway that enables cancer cells to convert cancer cell-derived cGAMP (an immune stimulatory molecule produced by cGAS upon encountering cytosolic dsDNA) into adenosine (an immune suppressive molecule when present in the extracellular milieu). This occurs through the activity of two extracellular enzymes (ectonucleotidases) ENPP1 and NT5E/CD73 which are induced in response to CIN (Li et al. Cancer Discovery, 2021). This work identifies key therapeutic and immune vulnerabilities in chromosomally unstable tumors. More broadly, we are interested in dissecting tumor-intrinsic and extrinsic mechanisms that enable cancer cells to tolerate and co-opt chronic inflammatory signaling to acquire metastatic and drug-resistant traits. Such an understanding would pave the way for strategies that aim to exploit CIN-induced inflammation as a therapeutic vulnerability.
The cellular biology of cytosolic DNA
Ongoing errors in chromosome segregation present cancer cells with a unique challenge, namely the presence of genomic double-stranded DNA in the cytoplasm, where it does not naturally belong. Beyond the ensuing inflammatory signaling, how cancer cells process cytosolic DNA is not understood. Using biochemical and cellular tools, including super-resolution microscopy, we are interested in interrogating key pathways involved in the generation and processing of cytosolic DNA and their impact on genomic instability and cellular metabolism.
CIN and epigenetic reprogramming in cancer
Our recent work identified CIN as a driver of epigenetic alterations in cancer through an unexpected mechanism (Agustinus et al. Nature 2023). We found that when chromosomes undergo missegregation and transit into micronuclei, they undergo significant modifications in histone posttranslational modifications. Consequently, these changes lead to substantial disruptions in chromatin structure and contribute to heritable and persistent epigenetic abnormalities in the missegregated chromosomes. Interestingly, although micronuclei generally exhibit compact chromatin, we have identified certain promoters that display increased accessibility. This suggests that a small subset of genes might experience heightened, rather than reduced, transcriptional activity. These observations challenge the long-held belief that CIN primarily promotes transcriptional heterogeneity through genomic copy number alterations alone. It indicates that CIN can also act as a driver of epigenetic dysregulation in cancer, even in the absence of mutations in genes encoding epigenetic modifying enzymes. Our work highlights the significance of ongoing chromosome segregation errors in cancer evolution and proposes a potential mechanism for the generation of extra chromosomal circular DNA. This DNA is a notable characteristic of human cancer, exhibiting enhanced chromatin accessibility and hypothesized to arise from chromosome encapsulation within micronuclei.