I am a neurosurgeon-scientist and Chair of the Department of Neurosurgery. I have clinical expertise in the surgical management of brain tumors such as complex gliomas, meningiomas and skull base tumors. My lab focus is the study of human stem cells for regenerative purposes in the brain, but also as tools for the study of brain tumors.
Stem Cells as Tools for Brain Repair1
After establishing early on that neural stem cells derived from human ES cells were capable of integrating functionally and safely into a host rodent brain2, the lab embarked on two major regenerative projects:
Human ES grafts for Parkinson’s disease
This is a major collaboration between my lab and Lorenz Studer’s with a goal of obtaining FDA approval for a clinical trial2. The work benefits from a NYSTEM consortium grant, in which I serve as a co-PI and leader of the “in vivo” project.
Human ES-derived grafts for repair of radiation injury
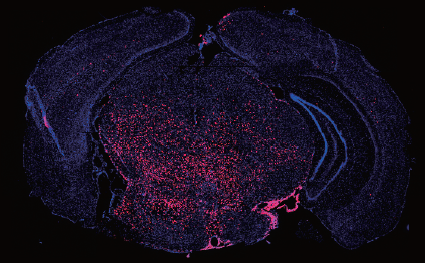
Radiation to the brain is a major contributor to poor quality of life among cancer survivors, yet it remains among the most potent tools in cancer therapy. Whole or partial brain radiation is a cornerstone of the treatment of many brain tumors, primary or metastatic, as well as a major component of prophylactic regimens in the treatment of leukemias. Young children are particularly vulnerable to the side-effects of radiation injury and often receive suboptimal radiation doses as a compromise between efficacy and safety. We have shown that radiation to the brain results in loss or significant depletion of the oligodendrocyte progenitor pool in the brain, both in a rat model we developed as well as in human brain tissue samples. We have also developed a scalable protocol for the efficient derivation of oligodendrocyte progenitors from human ES or iPS cells. The cells are well characterized by phenotype markers, gene expression profile and in vitro myelination. In a major effort over the past several years, we optimized rodent brain radiation to mimic the standard high dose fractionated regimen given to patients (50 Gy). The young rats develop cognitive and motor behavioral deficits within several months of treatment. Analysis of the rat brains shows depletion of the oligodendrocyte progenitor pool as well diffuse demyelination. Grafting resulted in structural repair shown histologically and by electron microscopy, as well as in a graft region-specific behavioral improvement. Forebrain grafts improved cognition while cerebellar grafts were required for amelioration of motor balance3. We plan to further optimize the cell culture and in vivo parameters in future pre-clinical studies.
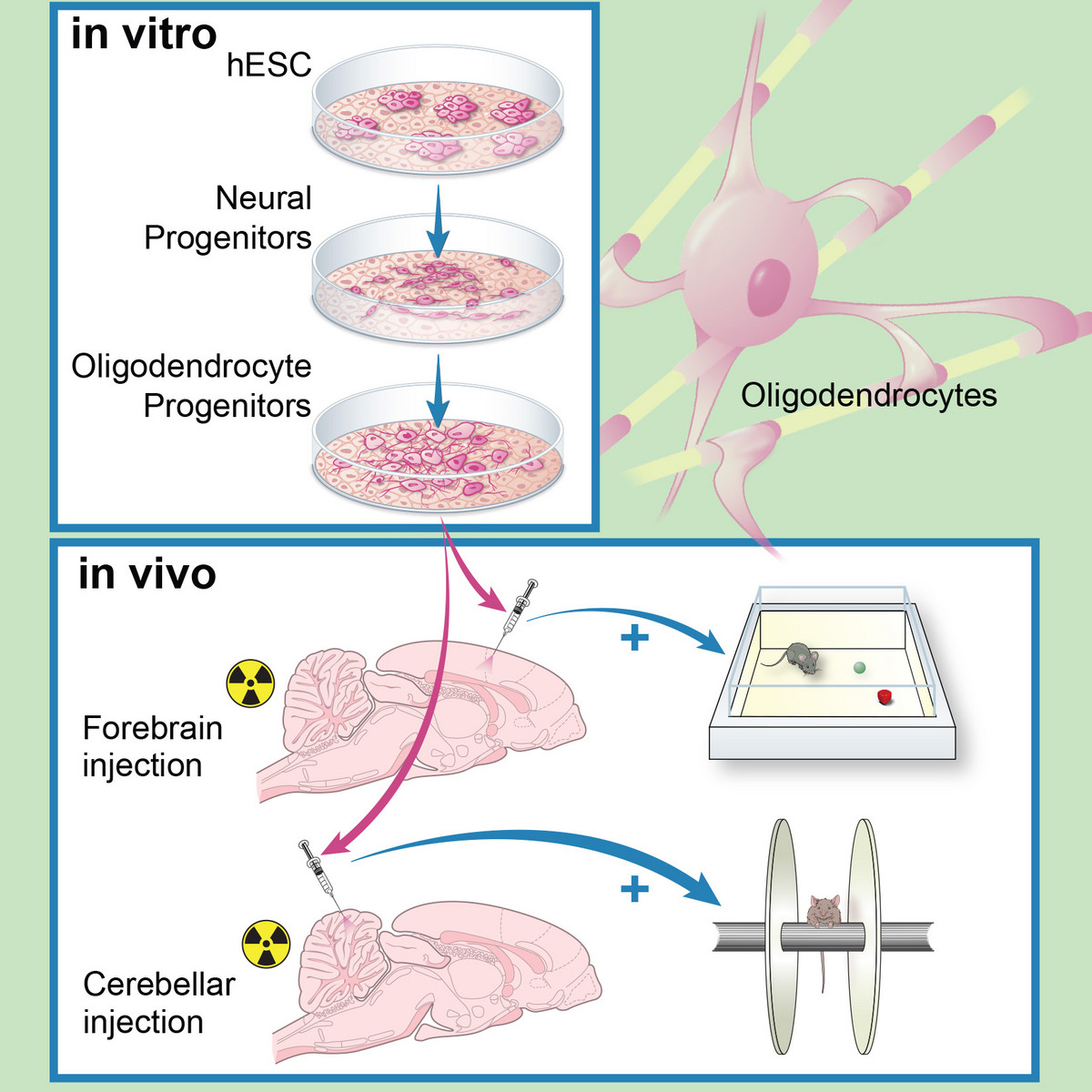
Human ES derived oligodendrocyte progenitors repair radiation-induced demyelination. Illustr. by Susan Weil.
Stem Cells as Tools to Model Human Cancer
Human ES cells as a platform for modeling brain tumors
Recent sequencing data have demonstrated that pediatric brainstem gliomas (DIPG) are characterized by mutations in histone genes, namely single amino acid substitutions in the tails of H3.3. While a role for post-translational histone modifications in cancer is well recognized, this was the first time mutations in a histone gene were identified as potential drivers in tumors. Key features of K27M-mutated DIPGs are the restricted developmental window during which they emerge [mean age at diagnosis is 8 years] and their specific midline location, which implicate a developmentally early and anatomically specific cell of origin. We postulated that human ES neural progeny might present an ideal candidate to model these tumors. In a recent publication in Science4 we demonstrate a cell context specific tumorigenic effect by H3.3K27M. The transformed cells were capable of initiating large tumors upon grafting in the brainstem of immunodeficient mice. Expression of H3.3K27M in neural precursors led to a developmental resetting to an earlier more primitive stem cell state, thus providing some insight into the biological basis of tumorigenicity by these tumors. In addition, we adapted hES derived neural precursors and their transformed counterparts to an epigenetic drug screen. Selecting for drugs with low IC50 and no negative impact on the normal cells, we identified and validated a menin inhibitor as a potential therapeutic agent against these tumors. Ongoing work is focused on the developmental of a therapeutic strategy for DIPGs as well as on dissecting the biology of histone mutations in tumors. We are also interested in expanding human pluripotent stem cells as a platform for modeling tumors.
Engineered human ES cell progeny form tumors in the brainstem, modeling pediatric tumors
Cellular heterogeneity in glioblastoma
Our lab has developed an interest in the study of glioblastoma from a stem cell and developmental perspective. We focused on the cellular heterogeneity and plasticity of the cell fractions that make up the tumor, but determined that it is imperative to study the tumors in their microenvironment. Using surgical tumor samples, we developed 3D explants that maintain tumor cytoarchitecture and blood vessels. The system allowed us to selectively remove endothelial cells (via toxin-conjugated antibodies) and identify their role in mediating Notch inhibition. We also demonstrated that a fraction of the abnormal tumor vasculature is neoplastic in origin (Nature 2010)5. More interestingly, we identified a small subfraction of tumor cells as the mediators of the transdifferentiation of tumor stem-like cells into endothelium. The work provides a window into the plasticity of tumor cells; these transitions from tumor to endothelium or perivascular cell are dynamic and the cells capable of such feats exhibit a different response to growth factor signaling. These data support a model of cellular hierarchy within solid tumors, based on cellular function rather than proliferative potential. We are further exploring the differential response to treatment by these heterogeneous cell populations.