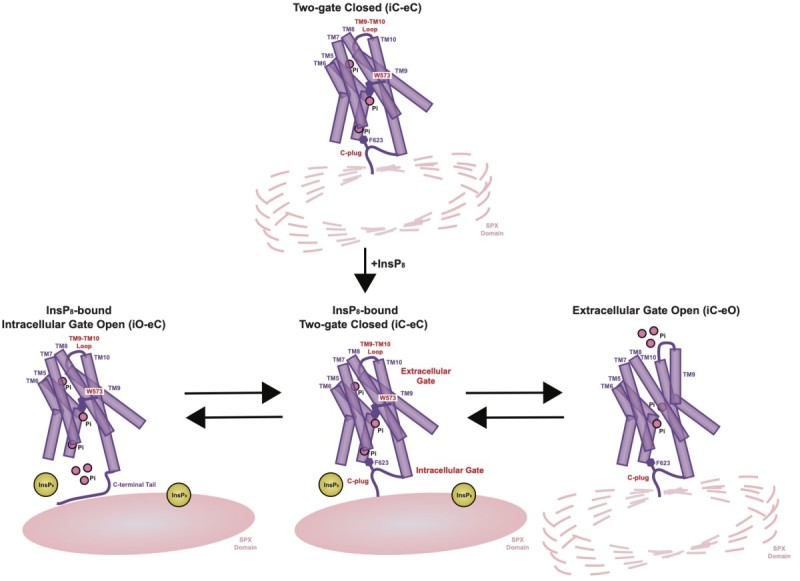
Inorganic phosphate (Pi) is one of the most essential nutrients for the maintenance of life. Pi is required for building cell membranes, DNA and RNA molecules, energy metabolism, signal transduction, and pH buffering. Pi transporters, including sodium-dependent Pi importers and the xenotropic and polytropic retrovirus receptor 1 (XPR1) Pi exporter (also known as SLC53A1), are major modulators of systemic and intracellular Pi concentrations. Recent work in the Diver lab has focused on XPR1. Uniquely, XPR1 senses and responds to Pi levels by binding to inositol pyrophosphate (InsP8), which also connects its activity to inositol polyphosphate signaling pathways within the cell. Inactivating mutations of XPR1 lead to brain calcifications, causing neurological symptoms that include migraine, movement disorders, psychosis, and dementia. Preventing Pi export through XPR1 from ovarian and uterine cancer cells leads to Pi toxicity and cell death. As such, inhibition of XPR1 is an attactive treatment option for these highly lethal cancers. Through the determination of cryo-EM structures of dimeric human XPR1 and its functional characterization, we have defined the substrate translocation pathway and delineated how InsP8 binding initiates the transport cycle. This work has significant implications for understanding basic biological processes in healthy and disease states, and for rationalizing disease mechanism and therapeutic intervention.