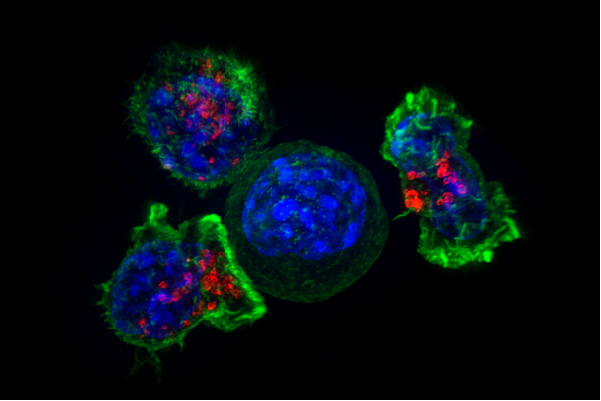
Cancer is smart, but your immune system is smarter.
At Memorial Sloan Kettering, we believe that immunotherapy is one of the most promising ways to treat, cure, and ultimately prevent cancer.
Immunotherapy was born at MSK more than a century ago. Since then our scientists have led the effort to develop new immune-based treatments for cancer. Our researchers have been at the epicenter of new discoveries in the field, and their work is bringing exciting new treatment options to people with cancer.
Patients who come to MSK for immunotherapy treatment benefit from unparalleled expertise in a field that our scientists pioneered.

Vinod Balachandran, MD, is a Hepatopancreatobiliary Surgeon at MSK who does laboratory research to identify new ways to use the immune system to fight pancreatic cancer.

Discoveries made in the lab of Immunology Program Chair Alexander Rudensky have changed the field of immunology.
- Our immunotherapy patients benefit from the close collaboration between our doctors and scientists.
- Discoveries in the lab are constantly being translated into new therapies for patients.
- We’re currently running nearly 100 immunotherapy-focused clinical trials.
- Our experts are seeking out new ways to help the immune system recover after a bone marrow transplant.
- Our science has already begun to change how melanoma, leukemia, and lung, bladder, and kidney cancers are treated.
MSK is home to a diverse group of scientists who study the immune system in all its complexity. Our laboratory scientists include many immunologists, geneticists, and cell biologists who explore the biology of immune cells and their interactions with tumors and infectious organisms.
Our physician-scientists are developing groundbreaking immune therapies that are helping to treat several forms of advanced cancer. They have played a lead role in developing and testing the immunotherapy drugs known as checkpoint inhibitors that “release the brakes” on the immune system, allowing it to mount a stronger attack against cancer. These drugs are transforming the way many cancers are treated, among them melanoma, lung, bladder, and kidney cancers.
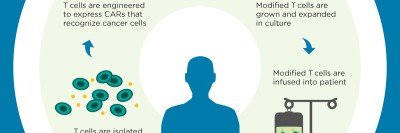
MSK scientists have also led the way in using chimeric antigen receptor (CAR) T cell therapy to treat leukemia and certain solid tumors. In this approach, immune cells from a patient are removed from the body, armed with new proteins that recognize cancer, and given back to the patient in large numbers.
MSK is a leader as well in bone marrow transplantation for children and adults with blood cancers such as leukemia.
Where Immunotherapy Began
Memorial Sloan Kettering’s roots in immunotherapy extend all the way back to 1893, when bone surgeon and cancer researcher William Coley began his work here on bacterial vaccines.
In the decades that followed, MSK scientists put cancer immunotherapy on firm scientific footing. Their many discoveries have helped make immunotherapy what it is today.
View our timeline of progress to see how MSK scientists have been at the forefront of immunotherapy research for more than 120 years.
Timeline Transcript
Timeline of Progress
In 1893, surgeon William Coley began treating cancer patients with a mixture of heat-killed bacteria (“Coley’s toxins") after noticing that patients with bacterial infections sometimes saw their tumors shrink. Experts today agree that many patients benefited from this treatment. Dr. Coley, who was for many years head of the bone tumor service at Memorial, is now considered to be the “Father of Cancer Immunotherapy.”
—Cancer immunosurveillance hypothesis proposed
Physician and future MSK president Lewis Thomas and immunologist Macfarlane Burnet propose the cancer immunosurveillance hypothesis — the idea that the immune system routinely patrols and protects the body from cancer. Only when these mechanisms fail does a person develop cancer. This idea is the intellectual foundation for the field of tumor immunology.
—Leukemia virus discovered
Virologist Charlotte Friend, while working at the Sloan Kettering Institute, identifies a virus that causes leukemia in mice. Her work lends strong support to the controversial idea that viruses can cause cancer. The virus she discovered is now known as the Friend leukemia virus.
—Studies of BCG launch modern era of tumor immunology
MSK scientists Lloyd Old and Donald Clarke, along with Baruj Benacerraf of New York University, publish a paper in Nature on bacillus Calmette-Guérin (BCG), a weakened form of the bacteria that cause tuberculosis. Mice injected with BCG developed resistance to the growth of implanted tumors. This is the first direct demonstration that the body’s immune defenses can be marshaled against cancer. Dr. Old is considered the "Father of Modern Tumor Immunology."
—Viral cause of nasopharyngeal cancer uncovered
Tumor immunologist Herbert Oettgen and colleagues identify antibodies to the Epstein-Barr virus in patients with nasopharyngeal cancer, suggesting a viral cause of this disease. Though a surprising discovery at the time, we now know that several cancers are caused by viruses.
—First successful bone marrow transplant from a sibling performed
Physician-scientist Robert Good, future director of SKI, administers the first successful bone marrow transplant from a sibling, permanently curing a boy who was born with severe combined immunodeficiency ("boy in the bubble syndrome"). Dr. Good also made groundbreaking contributions to our understanding of the thymus gland and the tonsils, two important immune organs.
—First bone marrow transplant from an unrelated donor performed
MSK pediatric oncologist Richard O'Reilly and SKI director Robert Good perform the first bone marrow transplant from an unrelated donor. This opens up the possibility of a transplant for the majority of patients, who lack a matched brother or sister. Bone marrow transplantation is a standard treatment today for some blood cancers. It is considered an immunotherapy because immune cells from the donor kill cancer cells in the recipient.
—Cancer-killing immune molecule discovered
Lloyd Old, Elizabeth Carswell, and colleagues identify tumor necrosis factor (TNF), a powerful immune system molecule that causes tumors to hemorrhage and die. TNF is produced by immune cells in response to bacterial products and is believed to underlie the anticancer effect of Coley’s toxins. It is the first immune system molecule shown to have a direct effect on cancer.
—Cancer antigens identified in melanoma
Pioneering immunologist Alan Houghton identifies cancer antigens in melanoma tumors, opening avenues to targeted immune therapies. Antigens are molecular markers found on the surface of cells that the immune system recognizes as foreign or dangerous. Drugs targeting these antigens are now a standard part of cancer treatment.
—Link between immune deficiency and cancer studied
Physicians Susan Krown and Bijan Safai study the relationship between immunodeficiency and Kaposi's sarcoma, a virally caused cancer, during the early years of the AIDS epidemic. Blood from Dr. Safai’s patients is used to develop the first antibody test for HIV.
—BCG immunotherapy approved by the FDA
BCG, a bacterial vaccine, is approved by the FDA for the treatment of bladder cancer, based on results of a clinical trial conducted by urologic surgeon Harry Herr and tumor immunologist Herbert Oettgen. BCG immunotherapy is still a primary treatment for non-muscle invasive bladder cancer.
—New cancer-associated antigen identified
Lloyd Old and colleagues identify a protein recognized by “killer” immune cells that is found in many cancers, but not in most normal body cells, with the exception of the testis. This “cancer-testis” antigen, NY-ESO-1, is now being tested in cancer vaccines and other immunotherapies.
—Treatment developed to enhance immune recovery
Physician-scientist Marcel van den Brink develops a treatment to improve immune recovery and healing following bone marrow transplantation (BMT), without aggravating graft-versus-host disease (GVHD). BMT patients are susceptible to dangerous infections following transplant. Treatments to boost immune recovery must be balanced against the possibility of GVHD, in which the donor cells attack normal tissues.
—Chimeric antigen receptor (CAR) T cells developed
Michel Sadelain, Renier Brentjens, and Isabelle Rivière of MSK develop genetically engineered T cells with a chimeric antigen receptor (CAR), now a powerful way to fight leukemia and other blood cancers. CARs are hybrid proteins made up of several immune molecules joined together. This team of investigators at MSK is the first to develop this technology, and continues to refine and optimize it to treat a variety of cancers. Dr. Sadelain won the William B. Coley award in 2012.
—Genetic switch for regulatory T cells discovered
Immunologist Alexander (Sasha) Rudensky shows that Foxp3 is the genetic switch controlling regulatory T cell development. Regulatory T cells are important immune cells that prevent unwanted or excessive immune reactions. Understanding how to selectively target particular regulatory T cells to enhance the immune response to cancer is a major goal of research in cancer immunotherapy. For his work, Dr. Rudensky won the William B. Coley Award in 2015.
—James Allison becomes Chair of Immunology at MSK
Immunologist James Allison joins MSK to help steer the clinical development of a new drug that blocks a molecule on immune cells called CTLA-4. Dr. Allison was the first to show, in 1996, that blocking CTLA-4 could cure cancer in mice. The new drug, called ipilimumab, opens up a whole new approach to cancer treatment geared toward “releasing the brakes” on the immune system. For his research, Dr. Allison won the William B. Coley award in 2005, the Lasker Award in 2015, and, along with Tasuku Honjo, the 2018 Nobel Prize in Medicine or Physiology.
—Ipilimumab FDA approved for treatment of melanoma
Ipilimumab (Yervoy®), an immune checkpoint inhibitor, is approved by the FDA for the treatment of advanced melanoma, based on clinical trials led by Jedd Wolchok. Patients at MSK began receiving ipilimumab in 2004, under the care of Dr. Wolchok. These patients are some of the earliest patients in the world to receive this drug, and a number of them are still alive today.
—Cancer immunotherapy voted “Breakthrough of the Year”
Cancer immunotherapy is voted “Breakthrough of the Year” by Science magazine. Among the promising approaches highlighted by the magazine are checkpoint blockade therapy and CAR T cell therapy — two areas pioneered by MSK scientists.
—Study shows why only some patients respond to immunotherapy
Physician-scientist Timothy Chan and colleagues publish a paper in Science that helps to explain why some people with lung cancer respond better than others to immunotherapies targeting the PD-1 immune checkpoint. Tumors with many genetic mutations seem to generate stronger immune responses than those with fewer. Two PD-1 immunotherapies, pembrolizumab and nivolumab, are now FDA approved for lung cancer.
—FDA approves first-ever immunotherapy combination
A combination of two immunotherapy drugs, ipilimumab and nivolumab, is approved by the FDA for the treatment of advanced melanoma, based on work by cancer immunologist Jedd Wolchok. Dr. Wolchok led the clinical trials that showed improved responses in patients receiving this combination versus ipilimumab alone. Many people believe that immune combinations will enable more patients to benefit from immunotherapy.
—FDA approves immunotherapy for advanced kidney cancer
The PD-1–blocking immunotherapy nivolumab is approved by the FDA for the treatment of advanced renal cell carcinoma, based on results of a large clinical trial led by MSK kidney cancer expert Robert Motzer.
—Parker Institute for Cancer Immunotherapy established
MSK is one of six founding centers of the Parker Institute for Cancer Immunotherapy, established through a $250 million grant from tech entrepreneur and philanthropist Sean Parker. The goal of the Parker Institute is to accelerate breakthroughs and drug development in cancer immunotherapy.
Programs and Collaborative Centers
Immunity science at MSK spans one academic program and five collaborative research centers, including the Parker Institute for Cancer Immunotherapy.
The Parker Institute, established by tech entrepreneur Sean Parker, brings together top researchers in the field from Memorial Sloan Kettering and five other founding partner institutions, with the aim of speeding the discovery and development of new immunotherapy treatment options.
Medical oncologist Jedd Wolchok has played a central role in developing and testing the immunotherapy drugs known as checkpoint inhibitors. Director Marcel van den Brink is a world-renowned expert on cell-based immunotherapy and bone marrow transplantation.

MSK patient Karen Koehler decided to give back after she was successfully treated with immunotherapy. Shortly after she completed CAR T cell therapy, she had her golden retriever, CJ, certified as a therapy dog. Read her story.

In the lab of immunologist Andrea Schietinger (center), whose work is focused on the interaction of T cells and cancer.